Probiotic use in preventing postoperative infection in liver transplant patients
Introduction
Liver transplantation has been widely practised for its success in prolonging survival and improving the quality of life for patients (1). In the U.S., over 40,000 patients annually progress to end-stage liver disease (ESLD), liver failure, and death. In addition, acute liver failure affects approximately 2,000 people every year in the U.S. Each year approximately 5,000 to 6,000 people in the U.S. with ESLD receive new livers with a 1-, 3-, and 5-year patient survival of 87%, 78%, and 73%, respectively (2).
Bacterial infection is a frequent complication following liver transplantation. The incidence of bacterial infection following liver transplantation has been reported to be 14.0-71.1% (3,4). Despite prophylactic administration of antibiotics the incidence of postoperative infections ranges from 10-30% in resectional surgery (5). Most infections are caused by bacteria of enteric origin (6). In spite of restricted use of prophylactic antibiotics, the emergence of antibiotic resistance has increased significantly. The gut microbial flora and mucosa are also affected by surgical trauma resulting in the gut barrier dysfunction and intestinal microbial imbalance. This may further aggravate systemic inflammation and depress immune function (7). All these factors contribute to an increased risk of postoperative infections and sepsis.
Probiotics can stabilize the intestinal barrier by stimulating epithelial growth, mucus secretion and motility as well as enhance innate immunity by inhibition of IL-10 and stimulation of secretory IgA, neutrophils and reduction of inflammatory cytokines (8). Furthermore, administration of probiotics suppresses growth of potentially pathogenic microorganisms, e.g., E. coli and Enterobacteriaceae. It has been hypothesized by several authors that these characteristics can be used in a clinical setting of preoperative prophylaxis for reduction of postoperative infections (9). Preoperative antibiotic prophylaxis constitutes more than ten percent of antibiotic usage in surgery and a reduction could lead to a reduced pressure on development of antibiotic resistance.
Since studies on preoperative use of probiotics was performed in patients undergoing liver transplantation in Germany, there was a significant reduction in postoperative sepsis and wound infection rate in the group that received living probiotics: 13% vs. 34% and 48% with heat-killed lactobacilli and bowel decontamination, respectively. They also observed a shorter hospital stay, lower number of days in intensive care and a decreased use of additional antibiotics in the group that received supplementation of lactobacilli (10,11). Postoperative leukocyte count was lower in the lactobacilli group. The results of this study are impressive but mechanisms underlying the observed effects could not be clarified. No evaluation of intestinal mucosal floras was done.
Clinical experience with pre- and probiotics in surgical patients is limited. The reason for the striking reduction in postoperative infections is not clear. More studies are needed for the further evaluation of fibre and probiotics use in liver transplantation.
Objectives
This study is to assess fibre + probiotic use aimed at preventing bacterial sepsis and wound complications in patients undergoing liver transplantation.
Study methods
This is a pre and post study, mainly comparing the result of patients receiving fibre only in 2010 and fibre + probiotics in 2011. Exclusion criteria were decompensated renal insufficiencies (creatinine clearance <50 mL/min) and disorders with danger of aspiration, both contraindications for uninterrupted enteral nutrition. The study was approved by the local ethics committee, and all patients gave written informed consent before study entry. Criteria to stop the study were withdrawal of patient consent and occurrence of serious adverse events.
Patients’ complete medical history and clinical examination, analysis of laboratory parameters, and disease-specific further examinations were evaluated.
Serum prealbumin and body mass index were measured and calculated to evaluate the nutritional status. The patients with liver cirrhosis were classified according to the Child’s-Pugh classification.
Patients
There were a total of sixty-seven adult patients scheduled for liver transplantation were included in a public teaching hospital. From January to December 2011, 34 continuous patients following liver transplantation were put on fibre + probiotics. In retrospectively, from January to December 2010, 33 continuous patients were collected as a control group and they were only received fibre post operation. The incidence of bacterial infections was compared in patients receiving either fibre and lactobacillus or fibre only. Routine laboratory parameters, nutritional parameters and the cellular immune status were measured in postoperative days 1, 5 and 10.
Group A
A synbiotic composition of prebiotics and probiotics was administered twice daily via the feeding tube or orally. Each capsule contains 6 different probiotic strains and 27 billion organisms of beneficial bacteria.
-
Lactobacillus Acidophilus (LA-14) 15.5 Billion;
-
Lactobacillus Plantarum (LP-115) 5.0 Billion;
-
Bifidobacterium Lactis (BL-04) 2.0 Billion;
-
Lactobacillus Casei (LC-11) 1.5 Billion;
-
Lactobacillus Rhamnosus (LR-32) 1.5 Billion;
-
Lactobacillus Brevis (LBr-35) 1.5 Billion.
Enteral nutrition with a low-fiber formula was started after patient tolerant oral fluid and continued for at least 7 days. If the patient did not have sufficient oral intake on, enteral nutrition was further continued.
Group B
Patients received only the fibers instead of firber + probiotics.
Regimen of antibiotics and catheters
All patients received single-shot intravenous prophylaxis before operation. After that, antibiotics were only given in case of bacterial infection. If infections occurred, patients were initially treated empirically and then following resistance testing of the isolated bacteria.
Proton pump inhibitors (40 mg pantoprazole daily) were routinely supplied once daily during the whole study period.
During operation, all patients received a central line and a urinary catheter. These catheters were removed as soon as possible except in case of serious complications.
Analyzed parameters
Incidence, type of infections, and type of isolated bacteria, length of hospital stay, days on intensive care unit, and duration of antibiotic therapy were recorded. In addition, side effects of enteral nutrition were evaluated. The duration of antibiotic therapy was determined by counting the number of days on which the patients received antibiotic therapy. The single-shot antibiotic prophylaxis was excluded. Total length of hospital stay was defined as the period between day of operation and discharge.
To rule out differences in intraoperative and postoperative risk factors for infections and to avoid a bias, we analyzed relevant accompanying diseases, tumor stage, alcohol and nicotine abuse, antibiotic therapy 1 month prior to operation, operating time, and number of transfused units of blood and fresh frozen plasma intraoperatively and postoperatively. The following well-known noninfectious complications were specifically looked at: biliary fistulas, anastomotic leaks, intra-abdominal hemorrhage, and impaired kidney function. In addition, relaparotomies were also registered.
Laboratory values were measured preoperatively and on postoperative days 1, 4, and 8, including hematology, clinical chemistry and C-reactive protein.
Surveillance and definition of infection
Body temperature was measured twice daily. Bacterial cultures from urine, blood, wound, and intra-abdominal drainages were done in case of suspected infection and intra-abdominal smears were taken, if relaparotomies were performed. The respective specimens were cultivated on agar plates for aerobic and anaerobic bacteria. Lactobacilli were also specifically looked for. Differentiation of bacteria was performed by using routine clinical methods. Results of the cultures were reported to the clinicians, but only patients with clinical signs of infection plus positive cultures were treated.
The diagnosis of bacterial infection was based on fever (>38 °C), elevation of C-reactive protein, specific clinical symptoms of infection as shown below, and a positive bacterial culture.
Wound infections
Detection of pus in the wound and a positive bacterial culture.
Pneumonia
Fever, cough, dyspnea, reduced arterial oxygen, typical pulmonary infiltrate on chest X-ray, positive culture from sputum, or bronchoalveolar lavage.
Peritonitis, intra-abdominal abscess
Fever, intra-abdominal pus, positive bacterial cultures from intra-abdominal smears.
Sepsis
Fever, low arterial blood pressure, systemic inflammatory response, and positive bacterial blood cultures.
Urinary tract infection
Dysuria, leukocyturia, and a positive urine culture with >105 colony forming units/mL.
Joint empyema
Swelling, pus, positive bacterial cultures from smears.
Cholangitis
Fever, elevation of cholestatic enzymes, dilated bile ducts on ultrasound.
Statistical analysis
Statistical analysis was performed using SPSS 15. The t test, fisher’s and chi- square test was used to compare discrete variables. A P value <0.05 was regarded as statistically significant.
Results
Demographic data
There were 67 patients completed the study, with 34 in group A and 33 in group B. Age, gender, and Child’s-Pugh classification of cirrhosis were equally distributed between the two groups (Table 1). The operating time, amount of intra- and post-operatively transfused units of blood, fresh frozen plasma and albumin did not differ significantly between the groups. The mean laboratory values including nutritional parameters did not differ significantly throughout the groups.
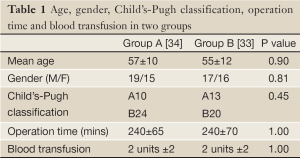
Full Table
Length of hospital stay and antibiotic therapy
There is no significant difference in the mean total length of hospital stay. Two patients in each group received antibiotic therapy during the month before operation. The duration of antibiotic therapy post operation was significantly shorter in group A than in group B (Table 2).

Full Table
Side effects of enteral nutrition
Enteral nutrition with probiotic and fibre was well tolerated in all patients. In group A, 2 out of 34 patients developed diarrhea and 3 out of 34 patients abdominal cramps; and in group B, 1 out of 33 patients had signs of diarrhea and 6 out of 33 patients abdominal distension and cramps. All side effects disappeared under temporary reduction in the amount of enteral nutrition.
Post-operative infections, mortality and other complications
Peri-operative mortality was 0% in both groups. Ten of group B patients (24%) developed bacterial infections, in total 10 infections. Wound infections (n=5), urinary tract infection (n=2), peritonitis (n=2), and pneumonia (n=1) were observed. All infections were treated with antibiotics. Most of the isolated bacteria were gut-derived with a predominance of Enterococci, Enterobacter, and E. coli.
In contrast, only 3 patients in the group A developed bacterial infections (11.8%), mainly wound infections. This difference was statistically significant (P<0.005). The infections were diagnosed at a mean of 9 (group A) and 8 days (group B) following surgery (Table 3).
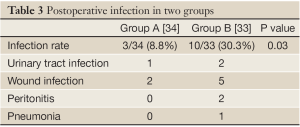
Full Table
In summary, in the analysis of 67 liver transplant recipients, 8.8% group A patients developed infections compared to 30.3% group B patients. The difference between groups A and B was statistically significant in both cases. In addition, the duration of antibiotic therapy was significantly shorter in the lactobacillus-group. Wound infection was the most frequent infections and enterococci the most frequently isolated bacteria. Fibre and lactobacilli were well tolerated in most cases.
Discussion
Despite advanced surgical techniques and broad-spectrum antibiotic prophylaxis and treatment, bacterial infection is still the most common cause of morbidity within the first 3 post-operative months in patients following liver transplantation (12). Post operative infection is the most frequent cause of death following liver transplantation even though deaths related to infectious diseases in nontransplant settings have steadily decreased. A recent study on 150 consecutive patients who underwent liver transplantation between October 1992 and January 1998. Complications included vascular (16.7% patients) and biliary (38.7%). Infections were bacterial in 92 (61.3%), fungal in 35 (23.3%), and cytomegalovirus in 9 (6%). The survival rates were 78% at 1 year, 68.7% at 5 years, and 58% at 10 years, only bacterial infections had a negative influence on patients’ survival (13).
In this study, prospective, randomized, early enteral nutrition supplemented with a mixture of probiotics and fibers significantly reduced the incidence of bacterial nosocomial infections following liver transplantation compared to only fibers. There were no significant differences between the groups regarding important risk factors for the development of infections like advanced age, accompanying liver or renal disease, malnutrition, a high number of intraoperatively and postoperatively transfused blood products, and unsuccessful operation. The majority of infections in this study occurred is the wound infection. There was no difference between the two study groups with regard to the length of duration of urinary catheters or number of patients with pre-existing diseases of the urinary tract. Therefore, probiotics are likely to be responsible for the reduction of these infections.
Although the prevention of mainly mild or moderate infections does not seem to be an important advantage, several additional positive effects were noted. As a consequence of the lower infection rates, the mean duration of antibiotic therapy was significantly shorter in group A. In addition, the length of stay on intensive care unit was not significantly different despite the higher rate of non-infectious complications in group A. Especially in high-risk patients who develop post-operative complications, this kind of prophylaxis could have the greatest benefit.
Besides prevention of bacterial translocation, synbiotics reduce and eliminate potentially pathogenic microorganisms, as well as various toxins and mutagens from urine and faeces, modulate innate and adaptive immune defence mechanisms, promote apoptosis, and release numerous nutrients, antioxidants, and growth factors from consumed fibers, functions that might also contribute to a reduction of surgical infections (14-16).
A recently published randomized, double-blind study of 55 cirrhotic patients with minimal hepatic encephalopathy with a similar study design and the same synbiotic combination as the present study, compared the effects of oral supplementation of the synbiotic combination during 30 days, to those of non-fermentable fiber (17). A significant decrease of venous ammonia and serum endotoxin levels, and prevention of cecal overgrowth with Escherichia coli and Staphylococcus spp. were observed. Furthermore, supply of the synbiotic composition led to reversal of minimal hepatic encephalopathy and improvement of liver function in approximately half of the patients. Interestingly, fermentable fibers alone were also effective in a substantial proportion of patients.
Although clinical results related to reduction of infections are evident in several studies, few attempts have been made to elucidate potential mechanisms. Potential reduction of bacterial translocation was studied in a few reports and results are inconsistent (18-20) and effect is minor, if present at all. Although most authors claim the potential effect of probiotics on gut microbiota, the absence of proper analysis makes it difficult to evaluate the clinical use of probiotics as prophylaxis of postoperative infections. Future studies in this field must more clearly address these issues. The length of administration, dose and type of probiotics used must be clarified, as well as how impact of probiotics on gut microbiota can be evaluated in patients.
Conclusions
Combined fibre and probiotics could lower the incidence of bacterial infections and short the duration of antibiotic therapy following liver transplantation in comparison to conventional nutrition. In contrast to antibiotics, it is relatively cheap and does not cause resistant strains or serious side effects. Because of limited study on prebiotics and probiotics in surgical patients, further clinical studies with larger patient numbers that also include measurement of special immune parameters are needed to confirm these preliminary results and to clarify the exact mode of action of pre- and probiotics.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Groth CG. Forty years of liver transplantation: personal recollections. Transplant Proc 2008;40:1127-9. [PubMed]
- Brown RS Jr, Lake JR. The survival impact of liver transplantation in the MELD era, and the future for organ allocation and distribution. Am J Transplant 2005;5:203-4. [PubMed]
- Li C, Wen TF, Mi K, et al. Analysis of infections in the first 3-month after living donor liver transplantation. World J Gastroenterol 2012;18:1975-80. [PubMed]
- Vera A, Contreras F, Guevara F. Incidence and risk factors for infections after liver transplant: single-center experience at the University Hospital Fundación Santa Fe de Bogotá, Colombia. Transpl Infect Dis 2011;13:608-15. [PubMed]
- Wade JJ, Rolando N, Hayllar K, et al. Bacterial and fungal infections after liver transplantation: an analysis of 284 patients. Hepatology 1995;21:1328-36. [PubMed]
- Dominguez EA. Long-term infectious complications of liver transplantation. Semin Liver Dis 1995;15:133-8. [PubMed]
- Hollenbeak CS, Alfrey EJ, Souba WW. The effect of surgical site infections on outcomes and resource utilization after liver transplantation. Surgery 2001;130:388-95. [PubMed]
- Cainelli F, Vento S. Infections and solid organ transplant rejection: a cause-and-effect relationship? Lancet Infect Dis 2002;2:539-49. [PubMed]
- Kinross JM, Markar S, Karthikesalingam A, et al. A meta-analysis of probiotic and synbiotic use in elective surgery: does nutrition modulation of the gut microbiome improve clinical outcome? JPEN J Parenter Enteral Nutr 2013;37:243-53. [PubMed]
- Engelhard D. Bacterial infections. In: Bowden RA, Ljungman P, Paya CV. eds. Transplant infections. Philadelphia: Lippincott- Raven, 1998:153-215.
- Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant 2005;5:125-30. [PubMed]
- Yang YM, Tian XD, Zhuang Y, et al. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol 2005;11:2456-61. [PubMed]
- Sintra SN, Tomé L, Cipriano MA, et al. Long-term outcome of the first 150 liver transplant recipients: a single-center experience. Transplant Proc 2013;45:1119-21. [PubMed]
- Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med 2003;31:598-607. [PubMed]
- Lidbeck A, Overvik E, Rafter J, et al. Effect of Lactobacillus acidophilus supplements on mutagen excretion in feces and urine in humans. Microb Ecol Health Dis 1992;5:59-67.
- Reid G, Jass J, Sebulsky MT, et al. Potential uses of probiotics in clinical practice. Clin Microbiol Rev 2003;16:658-72. [PubMed]
- Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39:1441-9. [PubMed]
- McNaught CE, Woodcock NP, MacFie J, et al. A prospective randomized study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut 2002;51:827-31. [PubMed]
- Anderson AD, McNaught CE, Jain PK, et al. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 2004;53:241-5. [PubMed]
- Reddy BS, Macfie J, Gatt M, et al. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg 2007;94:546-54. [PubMed]


