Laparoscopic resection for hepatocellular carcinoma: comparison between Middle Eastern and Western experience
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer. Currently, the treatment of HCC is multidisciplinary but surgery remains the gold standard. HCC can be treated by either liver resection or liver transplantation (LT). Resection can precede LT according to different strategies such as primary therapy, patient selection for LT based on tumor histology or as a bridge treatment before LT (1). In this context, the development of minimally invasive surgery has led to an increase in laparoscopic liver resection (LLR) for HCC in which the possibility of repeat surgery is normally accepted.
The LLR technique could be divided into three main categories based on the Louisville statement, i.e., pure laparoscopy, hand-assisted laparoscopy, and the hybrid technique (2). Pure laparoscopy involves the performance of the entire liver resection using laparoscopic ports only. The hand-assisted approach is defined as laparoscopy with the addition of a hand-port placed to facilitate the procedure, and the hybrid technique is when the operation is started laparoscopically to mobilize the liver, followed by a mini-laparotomy to complete parenchymal transection. Today, in patients with a solitary HCC <5 cm in the anterior segment, with no evidence of an extrahepatic tumor burden, in case of compensated liver disease with absence of significant portal hypertension, LLR is considered a safe and feasible treatment option (2,3). In addition, due to improved laparoscopic instruments and increasing surgical experience, the technical difficulty of LLR is slowly being overcome. Henceforth, series have reported LLR of lesions located in posterior superior segments with good results (4,5). A number of advantages have been recognized when comparing LLR to open liver resection (OLR) from case-matched analyses, including reductions in postoperative pain, less operative morbidity, and shorter length of hospital stay (6-10).
The purpose of this review is to provide a thoroughly detailed description of reported studies about LLR for HCC in the literature in recent years. Specific emphasis will be placed on the comparison between Middle Eastern and Western experience with regards to resection types, technical approaches, postoperative course, and outcomes.
Methods
A literature search was performed using PubMed, Scopus, and Web of Science (WoS) from cited English and Chinese publications. Data collection was performed until October 2013. Search phrases were “laparoscopy”, “liver resection”, and “HCC”. Manual cross-referencing was performed for all titles and abstracts, and relevant references from selected papers were reviewed. Publications with fewer than 15 cases, case reports, abstracts, letters, editorials and expert opinions were not considered for the drafting of the study. Review articles and meta-analyses were considered for the study. When there was more than one publication from the same team and/or authors, only the last publication in chronological order was considered for the study. Should a publication be written in Chinese, a translation was carried out, as faithful as possible, with the help of translators as native speakers.
Tables have been drawn up based on the geographical origins of authors and divided into Middle Eastern and Western experience. The results of the meta-analysis were not included in the tables.
Three reviewers (TP, DS, PP) independently considered the eligibility of potential publications and archived the following parameters from each study, namely first author, study design, number of patients, laparoscopic liver technique (pure laparoscopic, laparoscopic hand-assisted, laparoscopic assisted open), patient characteristics (age, gender, presence of cirrhosis, Child-Pugh score), size and number of tumors, location of tumor, type of resection (i.e., minor resection: ≤2 segments, major resection: ≥3 segments), associated resections, conversion rate, operative outcomes (operative time, blood loss, number of patients requiring transfusion, number of units of packed red blood cells (PRBCs), use and duration of portal clamping), postoperative outcomes (hopital stay, mortality and morbility [general and specific: hemorrhage, ascites, biliary collection, liver failure), and oncologic results [surgical margins, overall survivall (1-3-5 years) and percentage of recurrence].
Results
Studies included in the analysis
There were 593 relevant papers in the initial search. After eliminating case reports, abstracts, letters, duplicates and studies where it was impossible to recover the data of HCC only, 24 remaining original studies with more than 15 patients were analyzed. There were 11 studies from the Western world (11-21) including two multicenter series (12,18), and 13 studies from the Middle Eastern world (4,22-33), and 4 meta-analyses from Chinese institutions (6-9).
Western experience (Table 1)
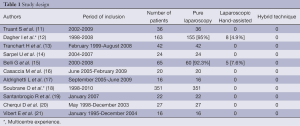
Full table
All 11 publications were retrospective analyses: four retrospective case-matched studies comparing LLR vs. OLR (11,13,14,17), two multicenter series from the French experience performed between 1998 and 2010 (18), and a European experience which included the databases of three European academic liver surgical centers (12). The other five series originated from a monocentric experience with minimally invasive approaches of LR: one series reported LLR in benign conditions and malignant tumors (21), and four series reported the feasibility of LLR in HCC (15,16,19,20).
Middle Eastern experience (Table 2)
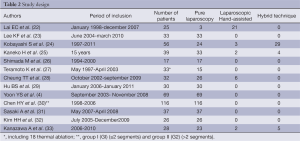
Full table
All 13 publications were retrospective analyses: only four studies were designed to compare the results of LLR versus OLR (28,29) including two retrospective case-matched analyses (23,32). The other 9 publications were monocentric experiences.
Selection criteria and type of laparoscopic approaches
Western experience
In three studies (12,13,15), selection criteria for LLR were well-compensated Child-Pugh Class A/B cirrhosis, esophageal varices ≤ grade 2, platelet count ≥80×109/L, small tumors less than 10 cm, without major vascular invasion, and ASA score not exceeding 3. Casaccia et al. (16) and Truant et al. (11) selected patients with platelet count ≥40×109/L, solitary lesion of ≤5 cm, and treatable via limited resection (<3 segments). In contrast, Vibert et al. (21) considered a disease with fewer than three nodules and no invasion of the portal convergence irrespective of the lesion’s diameter eligible for LLR. Aldrighetti et al. (17) and Santambrogio et al. (19) advocated the absence of previous major upper abdominal surgery as well as cardiac or respiratory failure.
In the Western surgical experience, only two series (12,15) reported the use of hand-assisted laparoscopy, with a percentage of total LLR of 92.3% and 95.1% respectively. No series reported any experience with the hybrid technique. All other experiences reported in the literature proposed a total laparoscopic approach associated with an incision to remove the surgical specimen.
Middle Eastern experience
In major series, lesions were ≤5 cm without any vascular invasion. For pure laparoscopic resection, Kobayashi et al. (24) reported a sufficient distance from major vascular branches, small tumors peripheral to the liver; for hand-assisted LLR, tumors located in the right posterior sector; and for hybrid resection, cancers not fulfilling any of the aforementioned criteria.
There were selective biological liver function tests such as albumin levels above 3.5 g/dL, bilirubin levels below 1.5 mg/dL, indocyanine green (ICG) retention at 15 min lower than 40%, and prothrombin time (PT) greater than 60% (26,33).
Five publications (38.4%) reported different techniques of LLR. In particular, Kobayashi et al. (24) compared hybrid with pure laparoscopic procedures as well as with open surgery. The hybrid procedure was applied to enlarge indications to minimally invasive surgery and represented about half of the cases in Kobayashi’s series. The percentage of laparoscopic hand-assisted procedures ranged from 5.1% to 84%.
Patients and tumors’ characteristics
Western experience (Table 3)
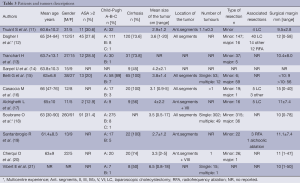
Full table
In all series, mean age was between 60 to 66 years with a predominance of male patients. The ASA score was >2 only in 30% of patients.
Fifty to one hundred percent of patients with cirrhosis presented a well-compensated chronic liver disease (Child-Pugh Class A). However, seven series reported patients with Child-Pugh Class B with a rate ranging from 3.2% to 22.7% (13,19). Only two series (12,18) reported their experience with Child-Pugh Class C patients.
LLR was recommended for lesions within 5 cm and with a mean size ranging from 2.7 to 6.5 cm. Vibert et al. (21) and Soubrane et al. (18) reported maximal tumor sizes of 18 and 17 cm respectively. The lesions were located only in anterior lateral segments more or less associated with segments VII and VIII in four studies (11,17,19,20). The most common type of LLR was a wedge resection or segmentectomy and left lateral sectionectomy. However, without considering multicenter studies, 13 major LLRs were performed (6%) (13,15,20). Radiofrequency ablation was associated for the treatment of intrahepatic lesions in three series (12,16,19).
Middle Eastern experience (Table 4)
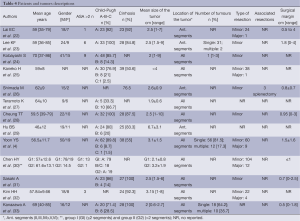
Full table
Only five studies (38.4%) reported an ASA score which was >2 in only 12.8% of cases.
Cirrhosis was present also from 50% to 100% of patients. Only two series did not describe the Child-Pugh status of the patients. Eight publications reported patients with Child-Pugh Class B cirrhosis ranging from 8.7% to 66.7%. In fact, Teramoto et al. (27) included 66.7% of Child-Pugh Class B patients with ICG retention rate at 15 minutes of 22.1±12.0%. Three series (4,25,30) reported their experience with Child-Pugh Class C patients. Chen et al. (30) reported patients with Child-Pugh Class B/C without distinguishing between the two different statuses.
Mean size of the tumor was less than 5 cm except for Hu et al. (29) who reported a mean tumor size greater than 6 cm. However, tumor size ranged from 0.6 to 9 cm. The lesions were located in all segments. Teramoto et al. (27) reported a thoracoscopic approach for posterior segments in five cases (S8=4, S7=1). Most of the resections were minor but 27 major LLRs (10.8%) were reported. The most common major liver resection was right hepatectomy. Only one central hepatectomy was performed by Yoon et al. (4).
Intraoperative and immediate postoperative outcomes
Western experience (Table 5)
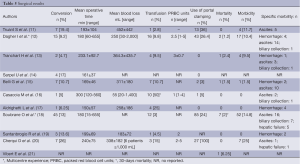
Full table
Mean operative time was between 150 to 300 minutes. In the four case-matched studies (11,13,14,17), there was no difference between LLR and OLR. In the series with major hepatectomy, maximal operative time ranged from 325.7 to 655 minutes.
Mean blood loss ranged from 55 to 452 mL. In two series, blood loss >1,000 mL was reported (12,16). Transfusion was required in all series, ranging from 2.8% to 50%. In case-matched studies, one study (17) reported a lower blood loss in the LLR group as compared to the OLR group (258±186 versus 617±433 mL; P=0.008). In contrast, the two other studies did not determine any difference between LLR and OLR (11,14). More than 50% of the series reported the use of a Pringle maneuver during resection. Cherqui et al. (20) reported 100% of intermittent portal triad clamping.
The conversion rate ranged from 5% to 19.4%. The most frequent reasons for conversion were bleeding during parenchymal transection, technical difficulties in exposure, and adhesions. In the four case-matched series (11,13,14,17), there was no difference in terms of surgical margins between LLR and OLR. Three cases of death were reported: one liver failure (13), one severe respiratory distress syndrome (15), and one cerebral infarction (21). The global morbidity rate ranged from 1.5% to 25%. Specific complications were divided into hemorrhage (2.4% to 25%), ascites (3.7% to 15.3%), and biliary collection (0.6% to 5%). A liver insufficiency was reported in two cases (18,20). Mean hospital stay ranged from 5.4 to 15 days. In all case-matched studies, LLR was statistically associated with a shorter hospital stay.
Middle Eastern experience (Table 6)
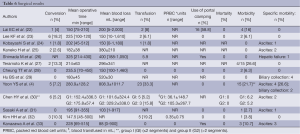
Full table
Mean operative time ranged from 147 to 325 minutes. In the two case-matched studies, there was no difference between LLR and OLR. In the five series with major hepatectomy, maximal operative time ranged from 210 to 500 minutes.
Mean blood loss ranged from 88 to 808 mL. In six series, blood loss >1,000 mL was reported (12,16). Save from two studies (28,33), transfusion was required, ranging from 1.8% to 19.2%. The two case-matched studies did not report any difference between LLR and OLR (23,32). Only three series reported the use of a Pringle maneuver during parenchymal transection (22,26,33).
The conversion rate ranged from 1.8% to 18.6%, and no conversion was reported in four series (7,8,31,33). The most frequent reasons for conversion were uncontrolled bleeding, and inadequate margin or poor localization (4,27,30). In five series (38.5%), the surgical margin was not reported. There was no mortality. There was no specific morbidity in five series (22,23,27,28,32). The main specific complication was ascites (1.7% to 26.6%). A biliary collection was reported in only two series (4,29) (10.7% and 13.3% respectively), and only one case of postoperative liver insufficiency was reported (26). Mean hospital stay ranged from 4 to 11.5 days. Three comparative studies statistically reported a shorter postoperative hospital stay following LLR versus OLR (23,28,32).
Long-term results: survival and recurrence
Western experience (Table 7)
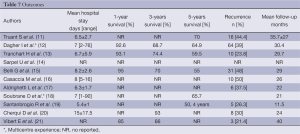
Full table
The 5-year overall survival rate was reported in five studies and ranged from 55% to 70%. Trancart et al. (13) reported no difference between LLR and OLR with a 1-, 3-, and 5-year overall survival rate of 93.1%, 74.4%, and 59.5% versus 81.8%, 73%, and 47.4% (P=0.25) respectively. No trocar-site recurrence was observed. The recurrence rate ranged from 21.4% to 50%. Comparative studies did not demonstrate any significant difference in terms of recurrence between LLR and OLR (11,13,14,17).
Middle Eastern experience (Table 8)
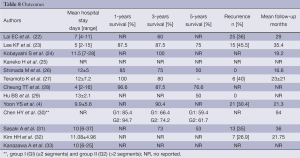
Full table
The 5-year overall survival rate was reported in six studies and ranged from 50% to 76.6%. Comparative studies did not demonstrate any significant difference in terms of overall survival and recurrence rate between LLR and OLR. Chen et al. (30) differentiated two groups of patients according to the type of resection (minor or major): the 1, 3 and 5 years were 85.4%, 66.4%, and 59.4% in the minor resection group, and 94.7%, 74.2%, and 61.7% in the major resection group respectively, without significant difference. No trocar-site recurrence was reported. The recurrence rate ranged from 26.9% to 45.5%, and two series (26,29) reported no recurrence.
Discussion
LLR for HCC is safe and feasible. Additionally, using a progressive approach, excellent outcomes can be obtained in the setting of underlying cirrhosis. Since the first reported case (34), an increasing number of series was published, and especially so since year 2000. Eight studies (four Middle Eastern and four Western ones) compared the benefits of the LLR versus the OLR approach but, to the best of our knowledge, a prospective randomized study has not been published yet (Table 9). In these different comparative studies, LLR can achieve survival equal to open hepatectomy in patients with HCC but with the benefit of less blood loss, less transfusion requirement, and a shorter hospital stay.

Full table
Selection criteria included tumor size and location as well as the severity of the underlying disease. It appears that the selection of patients is quite uniform in the Western experience. The Western most centers are French or Italian, and as reported in the multicenter study of Dagher et al. (12), centers use the same selection of patients and surgical techniques. On the opposite, in the Middle Eastern experience, selection criteria were less clear and authors reported that these criteria were similar to the ones of open surgery. In the Middle Eastern experience, for surgical evaluation, the ICG retention rate at 15 minutes represented the most reliable and faithful index of hepatic reserve. More Child-Pugh Class B and/or Class C patients were operated on in Middle Eastern series. No series used the Model for End-Stage Liver Disease (MELD) score for the selection of patients, currently used as a disease severity index of cirrhotic patients awaiting LT. However, the MELD score related with mortality and liver-related morbidities in HCC patients who underwent hepatic resection. A MELD score >8 represented the trigger for intensive treatment to improve patient outcome (35). In the Mayo clinic experience (36), a MELD score >9 was an independent predictor of perioperative mortality and long-term survival after multivariate analysis.
For some Middle Eastern surgeons, tumor location does not seem to be a selection criterion but the type of approach was different from pure laparoscopy. The Middle Eastern experience reported more hand-assisted or hybrid techniques. Huang et al. (37) reported a series of LLR with or without the hand-assisted approach and concluded that surgical results between hand-assisted and non-hand-assisted approaches were similar except for higher blood losses with the hand-assisted technique. The authors found that there was a higher use of hand-assisted LLR when liver cirrhosis was present, and less likelihood of using hand-assisted LLR when there was a superficial location of the tumor or lesion. In a comparative study (24), pure LLR was associated with lesser blood loss, and shorter skin incisions than in hybrid and open hepatectomy. Hybrid hepatectomy was associated with a longer operative time. It is probably for these few advantages and mainly for blood loss that Western surgeons prefer to use a pure laparoscopic approach. The hybrid technique in the Western experience was particularly described in cases of living donor right hepatectomies (38). The laparoscopic approach could be used in cases of HCC recurrence: a previous surgery and the grade of adhesions have not been subject to contraindications (39,40). Fewer adhesions represent an additional benefit of laparoscopic hepatectomy. This could well facilitate an easy reoperation for either a subsequent laparoscopic surgery or an open abdominal surgery to treat HCC recurrence or metastasis. LLR could be proposed as a bridge treatment before LT: LLR facilitated the LT procedure as compared to OLR in terms of reduced operative time, blood loss, and transfusion requirements (41).
With the benefit of experience, pure laparoscopy could be proposed for all tumor locations (42). Regarding the type of resection, the learning curve inherent to LLR reflects the attitude of the different teams for which the more accessible lesions are approached first prior to undertaking more difficult resections. LLR requires expertise in OLR, minimally invasive surgery, and laparoscopic ultrasonography. Resections in posterior and superior segments of the liver and major liver resections should be reserved for centers with a significant experience in laparoscopic liver surgery (42). In all groups, wedge resection and minor resection were more commonly performed. However, major hepatectomies such as right hepatectomies are increasingly proposed nowadays (43,44).
The resection margin is another factor that could well influence survival. A positive margin may have a profound influence on disease-free survival and long-term survival. The incidence of surgical margins <1 cm was reported in 62.5% of Middle Eastern articles. This result was set in contrast with “the dogma” in which a gross resection margin aiming at 2 cm provided better survival outcome than a narrow resection margin at 1 cm (45) for macroscopically solitary HCCs. However, in all case-matched control studies, survival rates, resection margins, and local recurrence rates following LLR were comparable to OLR. Laparoscopic intraoperative ultrasonography can be used to locate the tumor, making it possible to keep the intended margin. Another concern about laparoscopic resection of malignancies is the potential risk of tumor seeding. However, neither peritoneal carcinomatosis nor port-site recurrence were observed following HCC resection by laparoscopy. The use of a plastic bag to remove the specimen could help prevent this complication. The meta-analyses (6,8) have shown that LLR is comparable to OLR for HCC at 1-, 3- and 5-year overall survival. Consequently, LLR should be considered an acceptable alternative for the treatment of malignant liver tumors.
The results of the literature should be construed with caution due to several limitations. First, all data stem from non-randomized trials, and the overall level of clinical evidence is low. However, results have shown that LLR for HCC is superior to OLR in terms of perioperative results and does not compromise oncological outcomes. Consequently, LLR may be an alternative choice in the treatment of HCC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kluger MD, Cherqui D. Laparoscopic resection of hepatocellular carcinoma. Recent Results Cancer Res 2013;190:111-26. [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [PubMed]
- Yoon YS, Han HS, Cho JY, et al. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc 2010;24:1630-7. [PubMed]
- Cardinal JS, Reddy SK, Tsung A, et al. Laparoscopic major hepatectomy: pure laparoscopic approach versus hand-assisted technique. J Hepatobiliary Pancreat Sci 2013;20:114-9. [PubMed]
- Zhou YM, Shao WY, Zhao YF, et al. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci 2011;56:1937-43. [PubMed]
- Yao G, Cao J, Gu H, et al. Laparoscopic Hepatectomy versus Open Hepatectomy for Hepatocellular Carcinoma: A Meta-Analysis. Chin J Evid-based Med 2013;13:588-95.
- Parks KR, Kuo YH, Davis JM, et al. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB (Oxford) 2014;16:109-18. [PubMed]
- Yin Z, Fan X, Ye H, et al. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol 2013;20:1203-15. [PubMed]
- Kirchberg J, Reißfelder C, Weitz J, et al. Laparoscopic surgery of liver tumors. Langenbecks Arch Surg 2013;398:931-8. [PubMed]
- Truant S, Bouras AF, Hebbar M, et al. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc 2011;25:3668-77. [PubMed]
- Dagher I, Belli G, Fantini C, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg 2010;211:16-23. [PubMed]
- Tranchart H, Di Giuro G, Lainas P, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc 2010;24:1170-6. [PubMed]
- Sarpel U, Hefti MM, Wisnievsky JP, et al. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol 2009;16:1572-7. [PubMed]
- Belli G, Fantini C, Belli A, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhosis: long-term outcomes. Dig Surg 2011;28:134-40. [PubMed]
- Casaccia M, Andorno E, Domenico SD, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients. Feasibility of nonanatomic resection in difficult tumor locations. J Minim Access Surg 2011;7:222-6. [PubMed]
- Aldrighetti L, Guzzetti E, Pulitanò C, et al. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 2010;102:82-6. [PubMed]
- Soubrane O, Goumard C, Laurent A, et al. Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB (Oxford) 2014;16:357-65. [PubMed]
- Santambrogio R, Aldrighetti L, Barabino M, et al. Laparoscopic liver resections for hepatocellular carcinoma. Is it a feasible option for patients with liver cirrhosis? Langenbecks Arch Surg 2009;394:255-64. [PubMed]
- Cherqui D, Laurent A, Tayar C, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg 2006;243:499-506. [PubMed]
- Vibert E, Perniceni T, Levard H, et al. Laparoscopic liver resection. Br J Surg 2006;93:67-72. [PubMed]
- Lai EC, Tang CN, Ha JP, et al. Laparoscopic liver resection for hepatocellular carcinoma: ten-year experience in a single center. Arch Surg 2009;144:143-7; discussion 148. [PubMed]
- Lee KF, Chong CN, Wong J, et al. Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg 2011;35:2268-74. [PubMed]
- Kobayashi S, Nagano H, Marubashi S, et al. Hepatectomy based on the tumor hemodynamics for hepatocellular carcinoma: a comparison among the hybrid and pure laparoscopic procedures and open surgery. Surg Endosc 2013;27:610-7. [PubMed]
- Kaneko H, Tsuchiya M, Otsuka Y, et al. Laparoscopic hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Hepatobiliary Pancreat Surg 2009;16:433-8. [PubMed]
- Shimada M, Hashizume M, Maehara S, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc 2001;15:541-4. [PubMed]
- Teramoto K, Kawamura T, Takamatsu S, et al. Laparoscopic and thoracoscopic approaches for the treatment of hepatocellular carcinoma. Am J Surg 2005;189:474-8. [PubMed]
- Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 2013;257:506-11. [PubMed]
- Hu BS, Chen K, Tan HM, et al. Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J Gastroenterol 2011;17:4725-8. [PubMed]
- Chen HY, Juan CC, Ker CG. Laparoscopic liver surgery for patients with hepatocellular carcinoma. Ann Surg Oncol 2008;15:800-6. [PubMed]
- Sasaki A, Nitta H, Otsuka K, et al. Ten-year experience of totally laparoscopic liver resection in a single institution. Br J Surg 2009;96:274-9. [PubMed]
- Kim HH, Park EK, Seoung JS, et al. Liver resection for hepatocellular carcinoma: case-matched analysis of laparoscopic versus open resection. J Korean Surg Soc 2011;80:412-9. [PubMed]
- Kanazawa A, Tsukamoto T, Shimizu S, et al. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc 2013;27:2592-7. [PubMed]
- Wayand W, Woisetschläger R. Laparoscopic resection of liver metastasis. Chirurg 1993;64:195-7. [PubMed]
- Hsu KY, Chau GY, Lui WY, et al. Predicting morbidity and mortality after hepatic resection in patients with hepatocellular carcinoma: the role of Model for End-Stage Liver Disease score. World J Surg 2009;33:2412-9. [PubMed]
- Teh SH, Christein J, Donohue J, et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg 2005;9:1207-15; discussion 1215. [PubMed]
- Huang MT, Wei PL, Wang W, et al. A series of laparoscopic liver resections with or without HALS in patients with hepatic tumors. J Gastrointest Surg 2009;13:896-906. [PubMed]
- Koffron AJ, Kung R, Baker T, et al. Laparoscopic-assisted right lobe donor hepatectomy. Am J Transplant 2006;6:2522-5. [PubMed]
- Belli G, Cioffi L, Fantini C, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc 2009;23:1807-11. [PubMed]
- Kanazawa A, Tsukamoto T, Shimizu S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2013;20:512-7. [PubMed]
- Laurent A, Tayar C, Andréoletti M, et al. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2009;16:310-4. [PubMed]
- Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 2012;256:959-64. [PubMed]
- Tzanis D, Shivathirthan N, Laurent A, et al. European experience of laparoscopic major hepatectomy. J Hepatobiliary Pancreat Sci 2013;20:120-4. [PubMed]
- Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 2013;257:205-13. [PubMed]
- Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36-43. [PubMed]


