The multifaceted nature of retinoid transport and metabolism
Introduction
The term retinoid was coined by Sporn and colleagues in the mid-1970s (1). Retinoids comprise both natural and synthetic chemical species that bear a structural resemblance to all-trans-retinol, with or without the biological activity of vitamin A. Hence, vitamin A (by definition all-trans-retinol) is a natural retinoid species. Retinoids must be acquired from the diet either as preformed retinoid or as provitamin A carotenoids that are converted in the body to retinoid. Preformed retinoid is ingested primarily in the form of retinol or retinyl esters from animal food sources including dairy, fish, and meat (and with especially high abundance in liver). Provitamin A carotenoids are yellow, orange, and red pigmented compounds in plants, which may be converted within the body into vitamin A. It has been estimated that individuals in developing nations receive 70-90% of their retinoid from provitamin A carotenoids, whereas individuals in industrialized nations consume up to 75% of their total dietary retinoid as preformed retinoid (2).
Retinoids regulate important cellular processes including cellular proliferation, differentiation, and apoptosis, and hence they have roles in many essential physiological processes including the maintenance of immunity, barrier integrity, male and female reproduction, and embryonic development (3,4). These essential actions are thought to be mediated primarily by all-trans-retinoic acid and 9-cis-retinoic acid, which regulate transcription by serving as ligands for nuclear hormone receptors (5,6). All-trans-retinoic acid serves as the natural ligand for the three retinoic acid receptors (RARα, -β, and -γ); whereas 9-cis-retinoic acid is proposed to be a natural ligand for the three retinoid X receptors (RXRα, -β, and -γ) (7). Over 500 genes are reported to be responsive to either all-trans- or 9-cis-retinoic acid (8). There is growing evidence that retinoic acid also acts non-genomically, directly regulating intracellular signaling pathways (9).
Both insufficient dietary retinoid intake (resulting in hypovitaminosis A or vitamin A-deficiency) and excessive retinoid consumption (resulting in hypervitaminosis A or vitamin A-toxicity) pose challenges to human health. Retinoid-deficiency is the leading cause of preventable blindness in developing countries, affecting the most detrimental outcomes to pregnant mothers and young children (10). The World Health Organization estimates that 250 million preschool children, primarily in Africa and South-East Asia, do not receive adequate levels of dietary retinoid (10). Of these children, an estimated 500,000 become blind due to retinoid deficiency and an estimated 250,000 die each year due to increased risk of other severe illnesses, including diarrheal disease and measles (10). On the other hand, retinoid-toxicity is a growing cause for concern in the developed world since it has been associated with health problems including osteoporosis and bone fractures (2) and respiratory tract infections (11). High intake of retinoic acid is known to be teratogenic for both animal models and humans (12). A large study involving 22,748 pregnant women found that the ratio of newborns with defects associated with cranial-neural-crest tissue born to mothers who consumed more than 15,000 IU of preformed vitamin A daily compared to mothers who consumed less than or equal to 5,000 IU was 3.5 (12). Moreover, elevated levels of RBP (or RBP4), the sole specific blood transport protein for retinol, is proposed to be causally associated with the development of obesity-related metabolic disease, including impaired insulin responsiveness (and consequently type 2 diabetes), liver disease, and cardiovascular disease (13) (We note for the reader that with the completion of the Human Genome Project, the gene encoding RBP, the serum/plasma transport protein for retinol, was given the designation RBP4. Classically though, this protein has been known as RBP. Both RBP and RBP4 appear in current literature, with RBP4 being commonly used in the literature focused on its role in metabolic disease. However, both RBP and RBP4 refer to the same protein, encoded by the same gene. Throughout this article, we will solely use the term RBP4).
There is also controversy regarding the significance of intervention trials in which high doses of retinol and/or β-carotene were administered. Results from many trials suggest that retinol supplementation increases resistance to the severity of infection (14) and that retinol supplementation decreases mortality related to pregnancy (15). However, a few studies show that retinol supplementation did not benefit, and could have possibly worsened health outcomes. For example, the well-known Beta-Carotene and Retinol Efficacy Trial (CARET) and the Alpha-Tocopherol Beta-Carotene (ATBC) Cancer Prevention Study show that retinol and/or β-carotene supplementation increased risk of lung cancer to those already predisposed to lung cancer, i.e., smokers and asbestos-exposed workers (16-18). Thus, it is always important to remember that both retinoid deficiency and toxicity could pose health problems, so a balanced intake is needed. We propose that some of these findings and seemingly inconsistent outcomes mentioned above will be explained as we gain greater understanding of the complexities of retinoid metabolism and delivery.
This article is aimed at summarizing new insights gained in the last decade on retinoid transport in the circulation and uptake by tissues. One of our major goals for this article is to familiarize readers with this expanding and evolving understanding. Another of our goals is to provoke thought and conversation regarding how the different retinoid and carotenoid forms that can be measured in the circulation may be used to understand the role of retinoids in maintaining optimal human health.
Retinoid storage and metabolism in the body
Unlike most vitamins, retinoids are stored at relatively high levels within the body (19). The ability to store retinoids affords organisms an evolutionary benefit in times of dietary retinoid insufficiency, as retinoids in these stores may be mobilized in response to bodily demands. This provides a rationale for why it is important to understand the molecular processes underlying retinoid intake from dietary sources, transport within the circulation, storage in tissues, and mobilization from these stores.
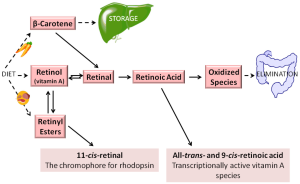
In the body, most retinoid exists primarily in one of two chemical forms—as either retinol or retinyl esters, both of which play key roles in the metabolism, storage, and delivery of vitamin A to tissues (19). Retinoid is transported in the circulation as retinol bound to RBP4. Retinol is also a precursor for retinoic acid formation, undergoing two enzymatically-catalyzed oxidizations to retinoic acid (19). Retinol can be esterified into retinyl ester through the actions of lecithin:retinol acyltransferase (LRAT), specifically, through transesterification of long chain fatty acyl groups present at the sn-1 position of membrane phosphatidyl choline to retinol. Retinyl esters are the body’s predominant retinoid storage form—healthy individuals store approximately 80-90% of the total retinoid present in their bodies as retinyl esters in the liver (20). Retinyl esters found in humans and rodents include primarily retinyl palmitate (which accounts for approximately 70-80% of total retinyl ester in the liver) along with retinyl oleate, retinyl stearate, and retinyl linoleate (21,22).
Within the liver, two distinct hepatic cell types are central to retinoid metabolism and storage: the parenchymal cells (or hepatocytes) and the non-parenchymal hepatic stellate cells (HSCs) (23,24). Hepatocytes constitute approximately two-thirds of all hepatic cells and approximately 90% of total hepatic protein (23,25,26). The large, relatively abundant hepatocytes are responsible for most of the liver’s metabolic processes—they are the cellular sites of dietary retinoid clearance in the liver and thereby responsible for dietary retinol uptake (27-32). The hepatocyte is also the major cellular site within the liver that is able to synthesize and secrete retinol bound to RBP4 (33). While hepatocytes are important for retinol uptake and mobilization, they account for only 10-20% of the total retinoid found in the liver (23,34,35). The remaining 80-90% of hepatic retinoid is found in the HSCs (23,34,35). HSCs contain characteristic large lipid droplets within their cytoplasm. Moriwaki et al. reported that lipid droplets purified from rat HSCs contained an average lipid composition consisting of 39.5% retinyl ester and that the lipid droplet composition responded markedly to changes in dietary retinol intake but not to changes in dietary fat intake, suggesting that the HSC lipid droplets are specialized for retinoid storage rather than neutral lipid (triglyceride or cholesteryl ester) storage (36). Adipose tissue, specifically the adipocyte, is another site for retinoid storage in the body, albeit to a lesser extent than hepatocytes and HSCs (37).
In addition to its role in retinoid storage, retinyl ester serves as the substrate for the formation of visual chromophore 11-cis-retinal, which is needed for visual pigment formation in the eye, and undergoes enzymatic recycling to retinol and subsequently retinyl ester in the visual cycle (19). Aside from its roles in storage and visual chromophore synthesis, retinyl ester has no other known biological roles (19). Retinyl esters are hydrolyzed to retinol to allow for its distribution throughout the body bound to RBP4 (19). Figure 1 summarizes the various retinol metabolites and their interconversions.
Longstanding view of retinoid transport in the circulation
Until recently, it was generally thought that the sole important retinoid delivery pathway to tissues involved retinol transported throughout the circulation bound to RBP4. Indeed, in the fasting human circulation, approximately 95% of the retinoids present exists as retinol bound to RBP4, with normal adult concentrations of approximately 2-4 µM (38-40). The retinol-RBP4 complex is secreted from the hepatocyte into the circulation to allow for retinol delivery to retinoid-dependent peripheral tissues, where it can be oxidized to retinoic acid (41). Retinol-RBP4 is found in the circulation as a complex with another protein, transthyretin (TTR). Binding to TTR stabilizes the retinol-RBP4 complex, thereby reducing renal filtration of RBP4 and allowing for RBP4 to be recycled after retinol is taken into cells (41). Exactly how retinol is taken up by cells from the retinol-RBP4-TTR complex is the subject of much current research and this will be discussed in the following section of this article.
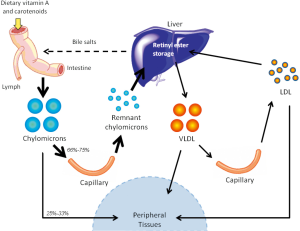
Another pathway for retinoid delivery to peripheral tissues—known for many years but the true significance of which has gone unrecognized—involves transport of dietary retinyl esters in chylomicrons (as depicted in Figure 2). Retinyl esters are packaged in chylomicrons along with other dietary fat (including cholesterol and triglyceride). Chylomicrons are secreted into the lymphatic system and eventually enter the general circulation through the thoracic duct (41). Before clearance by peripheral tissues, retinyl esters must first be hydrolyzed to retinol by lipoprotein lipase (LPL) (42). This newly formed retinol is thought to bind to cellular retinol-binding protein, type I (CRBPI) present in most peripheral tissues, which transports retinol within these tissues (43,44). After consumption of a retinoid-rich meal, the postprandial circulation may contain levels of retinyl esters as high as 5-10 µM, with exact concentrations directly depending on the quantity of retinoid consumed, while retinyl ester concentrations in the fasting circulation vary but are generally found within the 100-200 nM range (19). Studies in the 1960s in rodents established that approximately 66-75% of chylomicron retinyl ester is taken up by the liver, while the remaining 25-33% of chylomicron retinyl ester is delivered to peripheral tissues, bypassing the liver and its stores (21). A generalized summary of this classical understanding of retinoid transport is presented in Figure 3.
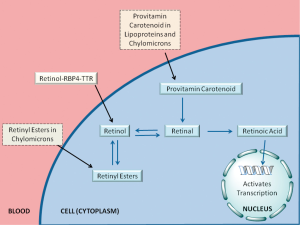
Global health experts may want to know the best or most accessible method for assessing retinoid status in human populations. The best indicator of retinoid nutritional status is certainly the hepatic concentration of total retinoids (retinol + retinyl esters) (51). However, the assessment of hepatic total retinoid levels requires either obtaining liver biopsies, which in our view is not feasible or even ethical, or using stable isotopes and mathematical models developed for this purpose, which may be expensive and technologically-sophisticated. Therefore, other indirect measures are commonly used, all of which involve measuring circulating retinoid levels in some context (possibly involving several blood draws). In our view, the most readily available assessment involves measuring serum retinol levels (51). As noted above, retinol-RBP4 accounts for the majority of retinoid in the fasting circulation and therefore perhaps affords the easiest assessment of the retinoid status of populations. However, two factors complicate this assessment. First, the liver defends blood retinol levels until the liver’s retinyl ester stores are completely depleted, after which retinoid levels in the blood plummet. Thus measuring serum retinol levels cannot identify individuals who appear to have a “normal” retinoid status but who actually have low hepatic retinyl ester stores and are in danger of hypovitaminosis A. Second, RBP4 and TTR levels in circulation may decrease due to infection or injuries. Upon injury or infection, the body activates an acute phase response, downregulating negative acute phase proteins, two of which include RBP4 and TTR (52). Therefore, a low serum RBP4 level may not accurately reflect retinoid status, as the low RBP4 levels result from the acute phase response to an infection or illness rather than low retinoid levels in the body. Although these two factors complicate the interpretation of measured serum retinol-RBP4 levels, they may not have significant effects on the assessment of retinoid status at the population level.
Recent insight into retinoid metabolism and transport in the circulation
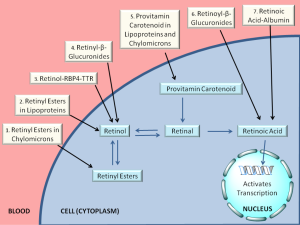
For the last ten or fifteen years, it has been increasingly recognized that a number of different forms of retinoid, in addition to retinol-RBP4, are found in the circulation at varying levels, depending on the dietary status of the individual. As summarized in Figure 4 and Table 1, these include retinyl esters transported in lipoproteins derived from the small intestine, in chylomicrons and chylomicron remnants, as well as from the liver, in very low density lipoprotein (VLDL) and low density lipoprotein (LDL); retinoic acid transport bound to albumin; and retinol and retinoic acid transported in the form of water-soluble retinyl- and retinoyl-β-glucuronides (19). We propose that all of these pathways can be important for assuring normal retinoid actions within the body and for contributing to tissue retinoid pools.
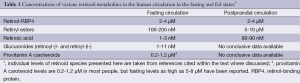
Full table
As an example of why this new understanding is significant, we would point to the following. For more than 30 years it has been known that consumption of foods rich in retinoids and provitamin A carotenoids is associated with a lessened risk of developing cancer. This finding is very reproducible across many populations. However, most studies that have attempted to demonstrate a link between blood retinol levels and cancer risk were unable to demonstrate such an association. The idea usually used to rationalize this apparent discrepancy is that foods rich in retinoids and provitamin A carotenoids contain many beneficial substances and it is not possible to tease out the effects of individual food components. This is certainly true. However, we would argue that solely relying on measurements of blood retinol levels themselves cannot determine dietary retinol sufficiency. As long as people consume a sufficient level of retinoid in the diet, blood retinol levels are maintained at a constant level by the actions of the liver. Thus, for this reason, we would argue that serum retinol levels cannot be directly correlated with incidence of cancer. Moreover, assessment of blood provitamin A carotenoid levels is also not useful for teasing apart this relationship since these can be enzymatically converted within tissues to retinoids, which is then used in situ to maintain retinoid-dependent functions including normal cell proliferation and differentiation. We take the view that the inverse relationship between dietary retinoids and provitamin A carotenoid intake and cancer reflects the delivery of these substances in chylomicrons and chylomicron remnants. Information that underlies this argument is discussed below.
RBP4
Until recently, research concerning retinoid transport has focused almost solely on retinol-RBP4 (53). Twenty years ago, it would have been predicted by most researchers that individuals lacking RBP4 would not be found since the absence of RBP4 would likely result in death at a very early age due to impairments to essential retinoid-dependent functions, like the maintenance of immunity and barrier function. However, research undertaken in the last fifteen years, both basic and clinical research, has demonstrated that other pathways for retinoid transport are also physiologically important. This idea is best evidenced in the existence of humans who completely lack RBP4 and are thus RBP4-deficient. These individuals display impaired vision and in some instances ocular defects, but do not exhibit other more severe signs of retinoid deficiency. In 1999, two female siblings living in Germany, with a known history of night blindness and slightly reduced visual acuity, were admitted to the hospital and found to have undetectable plasma RBP4 concentrations (53). Sequencing of the sister’s RBP4 gene revealed two unique point mutations, resulting in amino acid changes to the RBP4 protein. Sequence analysis showed that these siblings inherited two mutated alleles (resulting in different amino acid substitutions on the encoded proteins) (53). As a result, both affected siblings experienced night blindness and modest retinal dystrophy, presumably due to insufficient retinoid delivery to the eye, but both showed no potentially severe symptoms of retinoid deficiency, such as an impaired immunity (53). Another family, living in South Asia and exhibiting a retinal degeneration, showed a similar total lack of RBP4. Two affected siblings experienced visual defects, but showed no signs of xerophthalmia (54). Exome sequencing showed abnormal changes in the RBP4 gene of these two affected patients but not in their unaffected sibling (54). These two siblings who completely lacked RBP4 protein had undetectable levels of serum retinol, whereas an unaffected sister had a more normal level of serum retinol (~1 µM). For both studies, TTR levels were reported to be normal, indicating that the patients had reduced RBP4 levels due to mutations in RBP4 itself, rather than due to lack of TTR (53,54). Studies in animal models agree with these findings from clinical studies—mice lacking RBP4 are also generally normal, aside from an impaired vision phenotype (55,56).
In the last decade, considerable research interest has focused on a possible link between RBP4 and obesity, diabetes, and insulin signaling. In 2005, Kahn and colleagues reported a role for RBP4 in linking the development of obesity with impaired insulin responsiveness. These investigators reported that elevated serum RBP4 concentrations were associated with insulin resistance and proposed that this association may causally link obesity and the development of type 2 diabetes (57). This relationship has now been studied in many clinical settings by many independent laboratories and many have concluded that overexpression of RBP4 increases susceptibility for the development of insulin resistance (57). Thus, many researchers believe that increased RBP4 levels, owing to obesity, impairs insulin signaling and possibly contributes to development of type 2 diabetes and its related diseases (58-60). However, other researchers still challenge the notion that RBP4 is correlated with obesity or diabetes (61,62). This controversy in the literature remains to be resolved, but this research has important implications given that the increasing incidence of type 2 diabetes is now a major public health concern in most parts of the world.
RBP4 and stimulated by retinoic acid 6 (STRA6)
Another area of considerable recent research interest concerns how retinol is taken up by cells from retinol-RBP4 present in the circulation. Even though free retinol has the ability to diffuse through cell membranes, retinol in the circulation is bound to the RBP4 in the retinol-RBP4-TTR complex, thereby preventing its diffusion into cells (48). In 2007, a cell surface receptor for RBP4 was identified—this receptor was termed STRA6 (49,50). STRA6 is expressed in a number of tissues with high demand for vitamin A, especially the retinal pigmented epithelial (RPE) cells of the eye (50). STRA6 interacts with the RBP4 component of the retinol-RBP4-TTR complex and facilitates retinol uptake by the cell (48). The early literature suggested that mutations in or lack of the STRA6 gene in humans results in a very severe phenotype, including, but not limited to, mental retardation, congenital heart failures, and developmental abnormalities resulting in death at an early age (48).
The more recent literature suggests that the severity of phenotypes resulting from human STRA6 mutations is variable but always results in impaired eye development (45). Recent experimental studies show that mice lacking Stra6 are viable and generally normal, with the primary phenotype being an ocular one of impaired vision and eye development (45-47). Two of these recent studies also established that the total absence of Stra6 in mice does not significantly affect retinol or retinyl ester levels in adipose tissue, brain, heart, kidney, liver, lungs, muscle, pancreas, spleen, testis, or thymus, only those present in the eye (45,46). The other concluded that STRA6 is not the only pathway for retinol uptake into the eye (47). This raises a question as to how generally important STRA6 may be for mediating tissue uptake and accumulation of retinoid, especially outside of the eye (46). The recent literature also suggests a potential alternative role for STRA6 in insulin responsiveness (45,63,64).
Retinyl esters in chylomicrons
The older literature emphasized the importance of the retinol-RBP4 transport pathway for assuring retinoid delivery to tissues, although it had been known for some time that retinyl esters can be found in the postprandial circulation transported in chylomicrons (65). As noted above, although the retinol-RBP4 pathway is the major pathway for retinoid transport in the circulation, it is not an essential pathway. This is evidenced by the fact that humans who do not express RBP4 have impaired vision and possible eye defects, but are otherwise normal when they regularly consume a retinoid-sufficient diet (53,54,66). Therefore, there must be other mechanisms to account for retinoid transport in the body. As discussed earlier, relatively high levels of retinyl esters are transported in chylomicrons following a vitamin-A rich meal. Even though postprandial clearance of retinyl esters by peripheral tissues had been known fairly early on, researchers did not make the connection that this was an important route for vitamin A delivery to tissues. However, when it became clear that humans lacking RBP4 have relatively mild phenotypes, ones not resulting in mortality, this delivery pathway began to be recognized as a major contributor to retinoid delivery to tissues. In other words, the present understanding is that RBP4 exists to allow for retinoid stores to be mobilized from the liver, thus enabling the body to store vitamin A for use in times of dietary insufficiency. However, individuals can survive without RBP4, as long as they consume sufficient quantities of dietary retinoid. This is because postprandial retinoid is delivered effectively to extrahepatic tissues that are vitamin A-dependent, aside for the eye. At present it is not understood whether this process is regulated and responsive to tissue retinoid needs and/or intake levels or whether this is simply an unregulated process. It is, however, now generally believed that delivery of postprandial retinoid to tissues via chylomicrons allows for humans lacking RBP4 to survive and live relatively normal lives, albeit with diminished or impaired vision (53-56,65).
Retinyl esters in VLDL and LDL
In the mid-1970s, Smith and Goodman hypothesized that retinyl esters in the fasting circulation bound to VLDL and LDL were markers for retinoid toxicity, especially hepatic toxicity (67). They found elevated levels of serum retinyl esters in three patients taking in excessive levels of retinoids, and their studies were followed-up in animal models, involving rats fed large amounts of dietary retinoid (67). These investigators proposed that retinoid toxicity resulted from hepatic retinoid levels exceeding the mobilization and transport capacity of RBP4 (67). However, this notion may be incorrect. Recent studies do not support this hypothesis. A careful analysis of the serum retinyl ester concentrations in 6,547 adults who participated in the National Health and Nutrition Examination Survey (NHANES III) found no relationship between high fasting retinyl ester concentrations and liver dysfunction (68). This is contrary to the hypotheses put forward by Smith and Goodman that retinyl ester concentrations greater than 10% of total serum retinol (retinol + retinyl ester) concentration be considered as reflecting retinoid toxicity (68). However, because of the growing interest in developed nations regarding whether excessive dietary retinoid intake may result in subclinical retinoid toxicity, there is a need to understand the biologic significance of circulating retinyl esters present in VLDL and LDL.
Retinoic acid
Retinoic acid is the essential signaling form of retinoid that regulates transcription of many genes necessary in development. Low concentrations of retinoic acid are found in the circulation bound to albumin (69). Immediately following consumption of a retinoid-rich meal by human volunteers, specifically consisting of 100 grams of turkey liver, blood concentrations of all-trans-retinoic acid reached as high as 80-90 nM, suggesting that retinoids consumed in liver get converted into retinoic acid upon absorption (70). Blood concentrations are quickly restored to those at normal human fasting levels, ranging from 1-3 nM (70,71). The intestine seems to be a primary source of retinoic acid in the postprandial circulation (70). However, the details of retinoic acid biogenesis are complex and not completely understood (72). In other words, it remains unclear whether one or a few tissues contribute to retinoic acid pools in the fasting circulation or whether perhaps retinoic acid “leaks” into circulation from most or all tissues. We would note that retinol is enzymatically oxidized to retinal and then to retinoic acid, the transcriptionally active retinoid. As such, it would appear that this delivery mechanism for vitamin A parallels that of vitamin D or thyroid hormone, as all three involve relatively large serum concentrations of a transcriptionally-inactive precursor (retinol, 25-hydroxy-vitamin D, or T4, respectively) and relatively low concentrations of the transcriptionally active metabolite (retinoic acid, 1,25-dihydroxy-vitamin D, or T3, respectively). The similarity among these vitamins/hormones implies that we may refer to retinoic acid as a hormone, even though its biochemistry may not exactly resemble that of a hormone. There are no known enzymes that reduce retinoic acid to retinal, so excessive retinoic acid must instead be catabolized and eliminated from the body (19).
β-glucuronides of retinol and retinoic acid
Other circulating sources of retinoids for tissues are β-glucuronide conjugates of either retinol or retinoic acid. The late James A. Olson and his colleagues found retinyl- and retinoyl-β-glucuronides present in the human blood at levels ranging from 1-11 nM (73,74). These investigators proposed that retinyl- and retinoyl-β-glucuronides may be hydrolyzed by tissue β-glucuronidases to retinol and retinoic acid, respectively, which then serve as sources of retinoids for tissues (73,74). However, the role of β-glucuronides as sources of retinoid has not been systematically studied since Olson’s seminal work.
Provitamin A carotenoids
As introduced earlier, retinoids may be obtained from the diet as either preformed retinoid or provitamin A carotenoids, including the most abundant provitamin A carotenoid, β-carotene (75). Provitamin A carotenoids are absorbed intact by the intestine, and the efficiency of absorption of β-carotene from plant sources ranges anywhere from 5% to 65% in humans (76). Post-absorption, provitamin A carotenoids are transported to the blood in chylomicrons and their remnants, VLDL and LDL (75,77). Fasting human blood levels of β-carotene are generally within the range of approximately 0.2-0.5 µM for most well-fed people, but are reported for some people to reach as high as 5-8 µM (78,79). Increased dietary intake of β-carotene correlates with increased serum concentrations of this carotenoid (75). It remains unclear whether circulating carotenoids are specifically taken up by cells and tissues or whether they are simply taken up and processed along with other neutral lipids present in the lipoprotein particles.
It is important to note here that dietary β-carotene intake is considered a safe source of retinoid, compared to preformed dietary retinoid, because preformed dietary retinoid is well absorbed in humans whereas β-carotene conversion into retinoids is regulated by the intestine in response to retinoid status (76). However, it is clear that provitamin A carotenoids taken into the body by the intestine can be converted to retinoids by the enzyme carotene-15,15'-monooxygenase in a number of tissues including the liver and eye (80-83). This has led to the proposal that provitamin A carotenoids present in these tissues can be used as a local tissue source for generating retinoid. This hypothesis, we believe, has considerable merit, and we suspect the transport of provitamin A carotenoids in the circulation likely contributes to retinoid economy within tissues.
The future, what next?
Since the identification of vitamin A over a century ago, much has been learned about this fat-soluble vitamin. Until a little more than a decade ago, the understanding of retinoid transport in the circulation focused almost solely on retinol-RBP4. While retinol-RBP4 is certainly one major pathway for retinoid transport within the body and allows for retinoid to be stored in liver and later mobilized bound to RBP4, recent evidence shows that it is not the only one, nor is it essential for human or animal life. Retinoids are also transported via other mechanisms within the body, including delivery of retinyl esters in chylomicrons and their remnants, retinyl esters in VLDL and LDL, retinoic acid bound to albumin, retinol and retinoic acid derived from glucuronides, and retinoid synthesized from provitamin A carotenoids.
Despite the tremendous amount of new understanding gained over the last decade, much more remains to be learned about retinoid transport throughout the body. Moreover, this knowledge will likely be useful for understanding and assessing retinoid nutritional status, possibly affecting intervention programs. The following are just a few examples of unresolved challenges in understanding the biochemistry of retinoids, but these are by no means exhaustive:
- Is retinoid uptake from chylomicrons and their remnants regulated? It is known that the body regulates blood retinol levels, defending it at a set level, but it is unclear whether the body also regulates retinyl ester uptake into peripheral tissues. If levels of retinoids in the liver are low or high, will absorption of retinoids in the gastrointestinal tract or postprandial retinyl ester uptake by peripheral tissues change in response? How does the quantity of retinoid consumed in the diet affect the quantity that is taken up into peripheral tissues?
- What is the physiologic significance of retinyl esters in VLDL or LDL? Is this retinyl ester taken up and processed by tissues along with triglyceride and cholesterol or is it hydrolyzed to retinol and processed separately from these other lipids? Is the quantity of retinyl ester present in these lipoproteins regulated or responsive to dietary intake or tissue retinoid needs?
- How does one best assess retinoid toxicity? Can this be accomplished through a measurement of blood retinoid forms and their levels? Or are different or more sophisticated measures required?
- What is the importance of retinoids and RBP4 in metabolic disease development? The recent literature suggests roles for retinoids and/or RBP4 in the development of obesity, impaired insulin responsiveness, liver disease, and cardiovascular disease. Are these roles primary to disease development or do they modify disease progression and severity? Are retinoid homeostasis and/or RBP4 potential targets for therapeutic interventions?
- How is retinol bound to RBP4 taken up into cells and how is the process regulated? STRA6 has been identified as a high affinity receptor for RBP4 on the cell surface, but it is not expressed in the liver. It is unclear why humans with mutations in or lack of the STRA6 gene can have more severe phenotypes than humans with mutations in or lack of RBP4, as both are important proteins in the retinol-RBP4 pathway. Furthermore, are there other unidentified receptors that regulate retinol homeostasis in the liver and other tissues [as cited in (84)]?
- How does retinoic acid accumulate in tissues and how is retinoic acid taken up into tissues from the circulation? Which tissues may be responsible for biosynthesis and secretion of retinoic acid into the fasting circulation? And are cell surface receptors involved in taking up circulating retinoic acid?
Future research into retinoid transport and metabolism will need to focus on answering these questions. Findings from this future research will have important implications in the basic and clinical settings, as a more comprehensive understanding of retinoid metabolism and transport will allow investigators to better understand and develop efficient strategies for treating metabolic disease and for assessing nutritional strategies of at-risk populations.
Acknowledgements
The work from the authors’ laboratory that has been discussed in this chapter was supported by grants from the National Institutes of Health (RC2 AA019413, R01 DK068437, R01 DK079221 and R21 AA020561).
Disclosure: The authors declare no conflict of interest.
References
- Sporn MB, Dunlop NM, Newton DL, et al. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc 1976;35:1332-8. [PubMed]
- Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr 2006;83:191-201. [PubMed]
- Gudas JM, Sporn MB, Roberts AB. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AB, Goodman DS. eds. The Retinoids: Biology, Chemistry and Medicine. New York: Raven Press, 1994:443-520.
- Gudas LJ. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim Biophys Acta 2012;1821:213-21.
- Mangelsdorf DJ, Umesono K, Evans RM. The retinoid receptors. In: Sporn MB, Roberts AB, Goodman DS. eds. The Retinoids: Biology, Chemistry and Medicine. New York: Rave Press, 1994:319-50.
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 1996;10:940-54. [PubMed]
- Chawla A, Repa JJ, Evans RM, et al. Nuclear receptors and lipid physiology: opening the X-files. Science 2001;294:1866-70. [PubMed]
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 2002;43:1773-808. [PubMed]
- Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 2013;54:1761-75. [PubMed]
- Micronutrient deficiencies: Vitamin A deficiency. Available online: http://www.who.int/nutrition/topics/vad/en/. Accessed July 18 2013.
- Grotto I, Mimouni M, Gdalevich M, et al. Vitamin A supplementation and childhood morbidity from diarrhea and respiratory infections: a meta-analysis. J Pediatr 2003;142:297-304. [PubMed]
- Rothman KJ, Moore LL, Singer MR, et al. Teratogenicity of high vitamin A intake. N Engl J Med 1995;333:1369-73. [PubMed]
- Erikstrup C, Mortensen OH, Nielsen AR, et al. RBP-to-retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes Metab 2009;11:204-12. [PubMed]
- Sommer A. Vitamin A deficiency and clinical disease: an historical overview. J Nutr 2008;138:1835-9. [PubMed]
- West KP Jr, Katz J, Khatry SK, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. The NNIPS-2 Study Group. BMJ 1999;318:570-5. [PubMed]
- Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst 1996;88:1550-9. [PubMed]
- Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150-5. [PubMed]
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 1994;330:1029-35. [PubMed]
- O’Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology: Thematic Review Series: Fat-Soluble Vitamins: Vitamin A. J Lipid Res 2013;54:1731-43. [PubMed]
- Blaner WS, Li Y. Vitamin A Metabolism, Storage and Tissue Delivery Mechanisms. In: Dolle P, Niederreither K. eds. The Retinoids: Biology, Biochemistry and Disease. Wiley-Blackwell, 2014. In press.
- Goodman DW, Huang HS, Shiratori T. Tissue Distribution and Metabolism of Newly Absorbed Vitamin A in the Rat. J Lipid Res 1965;6:390-6. [PubMed]
- Tanumihardjo SA, Furr HC, Amedee-Manesme O, et al. Retinyl ester (vitamin A ester) and carotenoid composition in human liver. Int J Vitam Nutr Res 1990;60:307-13. [PubMed]
- Blaner WS, Hendriks HF, Brouwer A, et al. Retinoids, retinoid-binding proteins, and retinyl palmitate hydrolase distributions in different types of rat liver cells. J Lipid Res 1985;26:1241-51. [PubMed]
- Blomhoff R, Rasmussen M, Nilsson A, et al. Hepatic retinol metabolism. Distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J Biol Chem 1985;260:13560-5. [PubMed]
- Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis 2001;21:311-35. [PubMed]
- Friedman SL. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol Rev 2008;88:125-72. [PubMed]
- Blomhoff R, Helgerud P, Rasmussen M, et al. In vivo uptake of chylomicron [3H]retinyl ester by rat liver: evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc Nat Acad Sci USA 1982;79:7326-30. [PubMed]
- Blomhoff R, Holte K, Næss L, et al. Newly administered [3H]retinol is transferred from hepatocytes to stellate cells in liver for storage. Exp Cell Res 1984;150:186-93. [PubMed]
- Cooper AD. Hepatic Clearance of Plasma Chylomicron Remnants. Semin Liver Dis 1992;12:386-96. [PubMed]
- Blaner WS, Olson JA. Retinol and retinoic acid metabolism. In: Sporn MB, Roberts AB, Goodman DS. eds. The Retinoids: Biology, Chemistry, and Medicine. 2nd ed. New York: Raven Press Ltd., 1994:229-55.
- Redgrave TG. Chylomicron metabolism. Biochem Soc Trans 2004;32:79-82. [PubMed]
- Abumrad NA, Davidson NO. Role of the Gut in Lipid Homeostasis. Physiol Rev 2012;92:1061-85. [PubMed]
- Blaner WS, O’Byrne SM, Wongsiriroj N, et al. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta 2009;1791:467-73.
- Batres RO, Olson JA. A Marginal Vitamin A Status Alters the Distribution of Vitamin A among Parenchymal and Stellate Cells in Rat Liver. J Nutr 1987;117:874-9. [PubMed]
- Blomhoff R, Berg T, Norum KR. Distribution of retinol in rat liver cells: effect of age, sex and nutritional status. Br J Nutr 1988;60:233-9. [PubMed]
- Moriwaki H, Blaner WS, Piantedosi R, et al. Effects of dietary retinoid and triglyceride on the lipid composition of rat liver stellate cells and stellate cell lipid droplets. J Lipid Res 1988;29:1523-34. [PubMed]
- Tsutsumi C, Okuno M, Tannous L, et al. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem 1992;267:1805-10. [PubMed]
- Soprano DR, Blaner WS. Plasma retinol-binding protein. In: Sporn MB, Roberts AB, Goodman DS. eds. The Retinoids: Biology, Chemistry and Medicine. 2nd ed. New York: Raven Press Ltd., 1994:257-81.
- Chuwers P, Barnhart S, Blanc P, et al. The protective effect of beta-carotene and retinol on ventilatory function in an asbestos-exposed cohort. Am J Resp Crit Care Med 1997;155:1066-71. [PubMed]
- O’Byrne SM, Wongsiriroj N, Libien J, et al. Retinoid Absorption and Storage Is Impaired in Mice Lacking Lecithin:Retinol Acyltransferase (LRAT). J Biol Chem 2005;280:35647-57. [PubMed]
- Combs GF. eds. The Vitamins: Fundamental Aspects in Nutrition and Health. San Diego, CA: Academic Press, 1992.
- Blaner WS, Obunike JC, Kurlandsky SB, et al. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J Biol Chem 1994;269:16559-65. [PubMed]
- Noy N, Blaner WS. Interactions of retinol with binding proteins: studies with rat cellular retinol-binding protein and with rat retinol-binding protein. Biochemistry 1991;30:6380-6. [PubMed]
- Ong DE, Newcomer ME, Chytil F. Cellular retinoid-binding proteins. In: Sporn MB, Roberts AB, Goodman DS. eds. The Retinoids: Biology, Chemistry and Medicine. 2nd ed. New York: Raven Press Ltd., 1994:283-318.
- Berry DC, Jacobs H, Marwarha G, et al. The Stra6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem 2013;288:24528-39. [PubMed]
- Terra R, Wang X, Hu Y, et al. To investigate the necessity of STRA6 upregulation in T cells during T cell immune responses. PLoS One 2013;8:e82808. [PubMed]
- Ruiz A, Jacobs MM, Klopfenstein M, et al. Retinoid content, visualo responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding rpotein receptor, STRA6. Invest Ophthalmol Vis Sci 2012;53:3027-39. [PubMed]
- Sun H, Kawaguchi R. The membrane receptor for plasma retinol-binding protein, a new type of cell-surface receptor. Int Rev Cell Mol Biol 2011;288:1-41. [PubMed]
- Kawaguchi R, Yu J, Honda J, et al. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007;315:820-5. [PubMed]
- Blaner WS. STRA6, a Cell-Surface Receptor for Retinol-Binding Protein: The Plot Thickens. Cell Metab 2007;5:164-6. [PubMed]
- Haskell MJ, Handelman GJ, Peerson JM, et al. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr 1997;66:67-74. [PubMed]
- Gruys E, Toussaint MJ, Niewold TA, et al. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B 2005;6:1045-56. [PubMed]
- Biesalski HK, Frank J, Beck SC, et al. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am J Clin Nutr 1999;69:931-6. [PubMed]
- Cukras C, Gaasterland T, Lee P, et al. Exome analysis identified a novel mutation in the RBP4 gene in a consanguineous pedigree with retinal dystrophy and developmental abnormalities. PLoS One 2012;7:e50205. [PubMed]
- Quadro L, Blaner WS, Salchow DJ, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 1999;18:4633-44. [PubMed]
- Quadro L, Hamberger L, Gottesman ME, et al. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 2005;146:4479-90. [PubMed]
- Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol 2011;165:703-11. [PubMed]
- Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356-62. [PubMed]
- Graham TE, Yang Q, Blüher M, et al. Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. N Engl J Med 2006;354:2552-63. [PubMed]
- Munkhtulga L, Nakayama K, Utsumi N, et al. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Human Genetics 2007;120:879-88. [PubMed]
- Janke J, Engeli S, Boschmann M, et al. Retinol-Binding Protein 4 in Human Obesity. Diabetes 2006;55:2805-10. [PubMed]
- Gómez-Ambrosi J, Rodríguez A, Catalán V, et al. Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clin Endocrinol (Oxf) 2008;69:208-15. [PubMed]
- Berry DC, Jin H, Majumdar A, et al. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci USA 2011;108:4340-5. [PubMed]
- Berry DC, O’Byrne SM, Vreeland AC, et al. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol Cell Biol 2012;32:3164-75. [PubMed]
- Goodman DS. Vitamin A and retinoids in health and disease. N Engl J Med 1984;310:1023-31. [PubMed]
- Seeliger MW, Biesalski HK, Wissinger B, et al. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest Ophthalmol Vis Sci 1999;40:3-11. [PubMed]
- Smith FR, Goodman DS. Vitamin A transport in human vitamin A toxicity. N Engl J Med 1976;294:805-8. [PubMed]
- Ballew C, Bowman BA, Russell RM, et al. Serum retinyl esters are not associated with biochemical markers of liver dysfunction in adult participants in the third National Health and Nutrition Examination Survey (NHANES III), 1988--1994. Am J Clin Nutr 2001;73:934-40. [PubMed]
- Gottesman ME, Quadro L, Blaner WS. Studies of vitamin A metabolism in mouse model systems. BioEssays 2001;23:409-19. [PubMed]
- Arnhold T, Tzimas G, Wittfoht W, et al. Identification of 9-cis-retinoic acid, 9,13-di-cis-retinoic acid, and 14-hydroxy-4,14-retro-retinol in human plasma after liver consumption. Life Sci 1996;59:PL169-77. [PubMed]
- Kurlandsky SB, Gamble MV, Ramakrishnan R, et al. Plasma Delivery of Retinoic Acid to Tissues in the Rat. J Biol Chem 1995;270:17850-7. [PubMed]
- Azaïs-Braesco V, Pascal G. Vitamin A in pregnancy: requirements and safety limits. Am J Clin Nutr 2000;71:1325S-33S. [PubMed]
- Barua AB, Olson JA. Retinoyl beta-glucuronide: an endogenous compound of human blood. Am J Clin Nutr 1986;43:481-5. [PubMed]
- Barua AB, Batres RO, Olson JA. Characterization of retinyl beta-glucuronide in human blood. Am J Clin Nutr 1989;50:370-4. [PubMed]
- Redlich CA, Grauer JN, Van Bennekum AM, et al. Characterization of carotenoid, vitamin A, and alpha-tocopheral levels in human lung tissue and pulmonary macrophages. Am J Resp Crit Care Med 1996;154:1436-43. [PubMed]
- Haskell MJ. The challenge to reach nutritional adequacy for vitamin A: beta-carotene bioavailability and conversion--evidence in humans. Am J Clin Nutr 2012;96:1193S-203S. [PubMed]
- Redlich CA, Chung JS, Cullen MR, et al. Effect of long-term beta-carotene and vitamin A on serum cholesterol and triglyceride levels among participants in the Carotene and Retinol Efficacy Trial (CARET). Atherosclerosis 1999;143:427-34. [PubMed]
- Faure H, Preziosi P, Roussel AM, et al. Factors influencing blood concentration of retinol, alpha-tocopherol, vitamin C, and beta-carotene in the French participants of the SU.VI.MAX trial. Eur J Clin Nutr 2006;60:706-17. [PubMed]
- Johnson EJ, Suter PM, Sahyoun N, et al. Relation between beta-carotene intake and plasma and adipose tissue concentrations of carotenoids and retinoids. Am J Clin Nutr 1995;62:598-603. [PubMed]
- Kiefer C, Hessel S, Lampert JM, et al. Identification and Characterization of a Mammalian Enzyme Catalyzing the Asymmetric Oxidative Cleavage of Provitamin A. J Biol Chem 2001;276:14110-6. [PubMed]
- Paik J, During A, Harrison EH, et al. Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem 2001;276:32160-8. [PubMed]
- Redmond TM, Gentleman S, Duncan T, et al. Identification, expression, and substrate specificity of a mammalian β-carotene 15,15'-dioxygenase. J Biol Chem 2001;276:6560-5. [PubMed]
- Wyss A, Wirtz GM, Woggon WD, et al. Expression pattern and localization of beta,beta-carotene 15,15'-dioxygenase in different tissues. Biochem J 2001;354:521-9. [PubMed]
- Alapatt P, Guo F, Komanetsky SM, et al. Liver retinol transporter and receptor for serum retinol-binding protein (RBP4). J Biol Chem 2013;288:1250-65. [PubMed]


