Perioperative nutritional support and fluid therapy in patients with liver diseases
Liver is the largest gland in human body, and it is also the center of nutrition metabolism that provides complex physiological function. The prevalence of liver dysfunction and malnutrition is reported to be 50-90% in patients with obstructive jaundice or moderate to severe cirrhosis (1). And liver dysfunction and nutritional deficiency are common among patients with indications for liver surgery. It has been suggested that the poor nutrition status before hepatic resection increase the risk of postoperative complications and/or mortality (2-6). Hepatic surgery significantly affects body’s metabolism and environment. Meanwhile, a high catabolic state caused by stress response will result in postoperative malnutrition, recovery delay and inhibition of the immune function, which leads to an increased risk for morbidity and mortality (1,7-9).
Therefore, essential nutritional support and nutrition therapy for patients with liver diseases undergoing hepatic surgery is very important, as it may improve their clinical outcome (4,10). However, considering the nutrient metabolism abnormalities in the liver during perioperative period, the nutritional support and fluid therapy could become complex. How to implement rational fluid therapy and nutritional support after liver surgery and effectively protect liver function should be attached sufficient importance.
According to the changes in nutrient status of the liver and metabolism function during perioperative period, there are several principles in nutritional support and fluid therapy we need to pay attention to.
Time
In the first 3 days after surgery, the body is in a stress state associated with a catabolic state, negative nitrogen balance. In this stage, it should be paid attention to maintaining major organs function. The peak of stress state should be taken into full consideration in nutritional support in order to avoid high calorie intake (11).
Enteral nutrition (EN) support
EN should be given high priority in patients receiving hepatic resection if conditions permit. The integrity of gastrointestinal function is not impaired in these patients, which gives us the opportunity to provide postoperative EN support at an early stage. Evidence shows that there are many benefits for patients receiving EN support perioperatively, such as helping to maintain the structure and functional integrity of the intestinal mucosal cells, protecting the intestinal mucosal barrier, reducing bacterial translocation and intestinal infection, improving the recovery of gastrointestinal function, and maintaining the body’s immune function. Reports have demonstrated the superiority of early EN in postoperative complication rates over parenteral nutrition (PN) (12-14). The American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines also support that EN is favored to preserve gut integrity and immune markers and to simplify glycemic management (15).
Reports showed that for patients undergoing hepatic resection, perioperative nutrition support, either enteral or parenteral, can reduce septic complications. Furthermore, postoperative early EN, especially The branched-chain amino acids (BCAA) -enriched nutrition, may prevent postoperative infections (10,12,13,16). How to choose different EN formulations depends on the degree of hepatic dysfunction and ascites. If patients do not have hepatic encephalopathy, general elemental diet or non-elemental diet will be good. Special elemental diet is preferable for liver failure with high BCAA in patients with hepatic encephalopathy, and EN preparations of low-sodium, high-calorie density are better for patients with more ascites. Pre-digested peptide preparations would be used in following condition: insufficiency exocrine pancreas, pancreatic drainage or intestinal bile shortage. And the concentration (from 12% increase to 24%) and infusion rate (from 50 mL/h increase to 100 mL/h) of nutrient solution should be increased day by day. The nutrient solution should not be more than 2,000 mL/d in total. Nowadays, most experts believe that PN combined with EN should be considered when EN cannot satisfy the energy needs (17).
Volume fluid of total parenteral nutrition (TPN)
The daily dose is approximately 50 mL/kg/day for adult patients requiring TPN. TPN volume should be no more than 3,000 mL for a 70 kg adult patient. If vomit, diarrhea, excessive drainage and other special circumstances occur, the amount of liquid could be appropriately increased, while the liquid intake should be limited depending on the status of patients with chronic renal insufficiency or heart failure (17,18).
Caloric intake
Low-calorie supply is encouraged since postoperative patients with liver disease are intolerant of an excess of nutritional support, while previous high-calorie supply (30 kcal/kg/d) has been abandoned. However, caloric standard of TPN for surgical patients is inconsistent in different professional fields and textbooks, ranging around 25-30 kcal/kg/d. Beth Israel Deaconess Medical Center (BIDMC) provides 21-23 kcal/kg/d for critically ill and postoperative patients to achieve good outcomes (19-21).
There is also a proactive downward trend for calories in China, but still no data on a wide range of surgical patients of moderate to severe degree. Researches, both domestic and abroad, show that excessive caloric intake for surgical patients would not reach the goal of nutritional support, but increase metabolic burden on patients, which causes hyperglycemic, liver damage and other metabolic disorders and complications. In fact, low caloric supplement in a short term after the surgery can avoid the metabolic burden and uncontrolled glucose regulation caused by excessive calorie supply exogenous, as well as decrease the incidence of related postoperative complications under traumatic stress and significant hormone antagonist stage, rather than change nutritional status. Peking Union Medical College Hospital explored the application of permissive inadequate intake firstly in China. The result confirmed that the permissive inadequate intake (18 kcal/kg/d) would not change the blood biochemistry and nutritional status of patients postoperatively in a short term (3-4 days), and it can avoid uncontrolled glucose regulation and benefit patients with successful rehabilitation. If the patient still cannot eat or is at the alternative stage of EN during the postoperative rehabilitation, the caloric supply should be adjusted to normal standard, 25 kcal/kg/d, to supply sufficient of calories to facilitate the rehabilitation of patients (22,23).
Fat emulsion choice
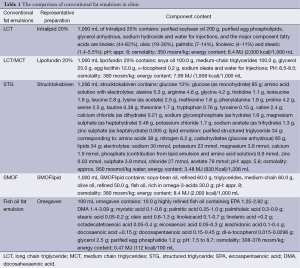
Patients with cirrhosis and other liver dysfunction are often accompanied by metabolic disorders, concomitant hypoalbuminemia and malnutrition. In clinical applications, long chain triglyceride (LCT) and medium chain triglycerides (MCT) are physically mixed at a ratio of 1:1 to meet the needs of essential fatty acids for human body, which is also helpful to the improvement of liver function and hepatocyte regeneration. In recent years, structured triglyceride (STG) has been widely used for its heat releasing stability and high safety. Compared with the conventional fat emulsion, STG fat emulsion is better in improving the nitrogen balance and more easily being removed by hepatocyte. Regarding the impact on monocyte-macrophage system, STG fat emulsion does not change the function of monocyte-macrophage system, and inhibits the human neutrophil migration (24-26).
In addition, the efficacy of the application of ω-3 fatty acids in critically ill patients has caused great concern. Clinical practice has proved that ω-3 fatty acids and other nutrients, such as immune glutamine, arginine, nucleosides and nucleotides, dietary fiber, and so on, can reduce the incidence of infection complications and promote wound healing. After liver resection, patients appear to have increased level of aminotransferase, caused by surgical trauma, damage of liver cell and liver ultrastructure, and release of inflammatory mediators. Studies have proved that, ω-3 fat emulsion can significantly improve liver function of patients after hepatic resection, by decreased IL-6 in peripheral blood and relief of HLA-DR -related inhibition. Another study suggests that ω-3 fatty acids can increase the liver perfusion, provide more oxygen, nutrients and metabolic substrates to hepatocytes (27-29).
The latest fat emulsion contains fish oil-SMOF that is composed of vitamin E—added soy long-chain fatty acids, medium chain fatty acids, olive oil, fish oil and vitamin E. This newly developed fat emulsion is mixed according to the ratio recommended by the U.S. National Institutes of Health (NIH) in 1999 which reduces the content of ω-6 fatty acids, increases the ω-3 fatty acids and provides sufficient of MUFA. Currently, on the basis of double-blind trial, it is believed that immune function can be best regulated by this ratio. Furst et al. pointed out that compared with the classic fat emulsion, SMOF fat emulsion is well tolerated in surgical patients. Due to its anti-inflammatory ability, it can help to regulate immune system and significantly shorten hospitalization time, but its efficacy in the application after partial liver resection has yet to be clinically validated (30-33).
The component contents of different conventional fat emulsions in clinic are compared in Table 1.
Full table
Amino acid choice
Currently, balanced amino acid preparation and specific amino acid preparation are widely used in clinic. The specific amino acid preparation includes compound amino acids for liver disease, kidney disease, trauma, children, and so on.
BCAAs—leucine, isoleucine, and valine—are essential amino acids. The higher content of BCAA in compound amino acids for liver disease can correct the disproportionality of BCAA, reduce aromatic amino acid that pass blood brain barrier, further relieve hepatic encephalopathy and may also have an effect on immunity and infections (34-37). Supplemental BCAA is commonly applied in patients with liver cirrhosis (38,39), especially in compensated cirrhosis (40,41) or hepatocellular carcinoma (42-46). Some studies reported that perioperative administration of BCAA to patients undergoing hepatic resection can increase albumin synthesis and quickly improve liver function during the early postoperative period (36,47-49).
It should be remembered that the supplement of serum albumin and BCAA after hepatic surgery is important. We should not only supply amino acid preparation rich in BCAA but also in other comprehensive amino acids to prevent disorder of protein synthesis.
Some issues on fluid theory during perioperative liver surgery
Albumin in liver surgery
In recent years, there are some new tendencies in applying of albumin among patients after abdominal surgery. Most evidence states that albumin is not directly involved in wound healing process of the body, and cannot improve the immune-related protein generation for immunity improvement either. After supplement, there will be 30% of albumin that will remain in the blood, indicating that it is mainly used for maintaining the colloid osmotic pressure and increasing capacity. The decrease of albumin after severe trauma and serious surgery can suggest the level of trauma degree in a certain extent (50,51).
Because albumin is almost entirely synthesized in the liver, liver resection surgery may lead to further dysfunction of albumin synthesis which reflects the trauma degree, especially in patients with impaired hepatic function. This decline may be related to temporarily blocked synthesis of albumin in the liver with obviously increased loss or decomposition. During tissue trauma, increased vascular permeability and albumin leakage lead to albumin redistribution. This is one of the most important reasons for the decline of serum albumin after surgery. According to a double blind, randomized controlled trial (SAFE study) that included 6,045 participants in Australia and New Zealand, the outcomes of resuscitation with albumin were not significantly better than saline in mortality, length of stay in the intensive care unit, length of hospital stay, duration of renal replacement therapy, or duration of mechanical ventilation (52).
However, more and more evidence suggests that preoperative hypoalbuminemia could become a single risk factor obviously related with the prognosis of severe patients (53-56). According to the authors’ experience, limiting albumin supplement could be considered when the preoperative serum albumin of liver function is normal in patients undergoing partial hepatectomy, instead of in patients with cirrhosis, liver function damage, a clear hypoalbuminemia, or undergoing multi-segmental hepatectomy. Perioperative supplemental albumin can supply the shortage of albumin caused by cirrhosis liver, correct hypoproteinemia, and reduce the probability of postoperative complications incidence. There is a clearly benefit of improving recovery in these postoperative patients.
Application of plasma
Plasma therapy is an important treatment during perioperative period. However, the application of plasma is not standardized and even misapplied in many cases, including the correction of hypoproteinemia, plasma protein supplements, improvement of colloid osmotic pressure; nutrition supplement; supplement of blood volume; increasing immunity; and improvement of liver function. Unnecessary application of the plasma wastes the scarce blood resources and also makes patients potentially exposed to risks of transfusion, such as increasing the chance of transfusion-related infections, transfusion-related lung injury, and transfusion-associated graft-versus-host disease (57,58).
Based on systematic analysis and literature review, we believe that perioperative plasma applications are limited into two conditions: firstly, improve plastic osmotic pressure if needed and maintain blood volume, especially for the patients who bleed much in a short period; secondly, supply coagulation factors and improve coagulation function.
Choice of crystalloid and colloid
The debate between crystalloid and colloid has existed for more than thirty years. Studies have showed that crystalloid mainly supply extracellular fluid and supplement the blood volume to ensure the volume of urine. High dose of crystalloid must be supplied if we want to maintain blood volume simply by crystalloid, but this therapeutic schedule often results in systemic edema, increased risk of pulmonary edema, cerebral edema and inadequate tissue perfusion. Meanwhile, electrolyte and acid-base disequilibrium such as hypernatremia, hyponatremia and alkalosis are often accompanied. The combined use of colloids, improvement of safety and effectiveness in volume therapy are the most basic problems in establishing clinical treatment scheme (52,59,60).
An evidence-based medical research in 1998 reported that application of albumin or plasma protein fraction may bring more probability of mortality than crystalloid or non-protein liquid. And a double blind, randomized controlled trial (SAFE study) showed similar mortality of resuscitation with albumin or saline (52). But a subsequent meta-analysis indicated that, compared with protein liquid, albumin can lower the morbidity and mortality rate (61). Latest studies showed that all artificial colloids (HES, gelatin, dextran) have renal side effects (62). With albumin as reference colloid, the anaphylactic reactions of artificial colloids are HES 4.51%, dextran 2.32%, gelatin 12.4% (63).
Based on the research results of large-scale monitoring of drug safety, albumin is the safest colloid and the incidence of side effect and serious adverse events are at an acceptable level (64). Reasonable selection of proportion of crystalloid and colloid, and infusion order according to individual conditions is necessary.
Liver protective therapy
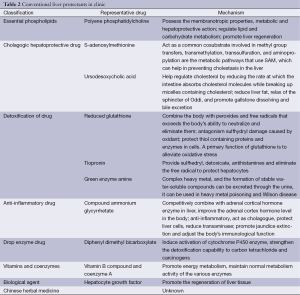
Hepatic protectant is the drug that could repair the damage of hepatocytes. There are many causes of hepatocytes injure, such as hepatitis and obstructive jaundice. During the treatment of protecting liver, we should eliminate the etiological factor at first, and then the treatment could include anti-viral therapy, surgery or removing the obstruction through interventional methods. There are a variety of liver drugs with different functions. By understanding the mechanism, properties and the application of these hepatic protectants would help hepatobiliary surgeons properly utilize these drugs. Clinical application of hepatic protectant should follow the principle of easy, safe, effective and low cost. The liver is the major organ that is responsible for the metabolism and detoxification of the drug in the body. Overdose would increase the burden of the liver, and some hepatic protectant with hepatotoxicity may cause liver toxicity and further damage hepatocytes. Hence, hepatic protectants with less liver toxicity should be chosen in clinic. The classification and mechanism of conventional hepatic protectants are shown in Table 2.
Full table
Conclusions
For perioperative therapy in patients with liver diseases, individualized nutritional support and fluid therapy plan should be adopted, on account of the need of the patient, disease mechanism characteristics, function of liver and the tolerance of the gastrointestinal tract. In order to restore EN and satisfy the needs of the patient for nutrition metabolism, we suggest use parenteral and EN sequential therapy for different perioperative stage and attach importance to the choice of fat emulsions containing medium chain fatty acids, olive oil and amino acids rich in BCAA. In the process of nutrition support and fluid therapy, reasonable selection of proportion and infusion order of crystalloid and colloid is necessary, and glucose level should be controlled strictly. With the treatment of hepatic protectants, albumin and plasma should be applied strictly in accordance with the indications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol 2012;10:117-25. [PubMed]
- Pikul J, Sharpe MD, Lowndes R, et al. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation 1994;57:469-72. [PubMed]
- Figueiredo F, Dickson ER, Pasha T, et al. Impact of nutritional status on outcomes after liver transplantation. Transplantation 2000;70:1347-52. [PubMed]
- Merli M, Nicolini G, Angeloni S, et al. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition 2002;18:978-86. [PubMed]
- Henkel AS, Buchman AL. Nutritional support in patients with chronic liver disease. Nat Clin Pract Gastroenterol Hepatol 2006;3:202-9. [PubMed]
- Tsiaousi ET, Hatzitolios AI, Trygonis SK, et al. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol 2008;23:527-33. [PubMed]
- Campillo B, Richardet JP, Bories PN. Enteral nutrition in severely malnourished and anorectic cirrhotic patients in clinical practice. Gastroenterol Clin Biol 2005;29:645-51. [PubMed]
- Gunsar F, Raimondo ML, Jones S, et al. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther 2006;24:563-72. [PubMed]
- Kalaitzakis E, Simren M, Olsson R, et al. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol 2006;41:1464-72. [PubMed]
- Fan ST, Lo CM, Lai EC, et al. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med 1994;331:1547-52. [PubMed]
- Nikolova-Todorova Z, Troić T. Effect of surgical trauma on patient nutritional status. Med Arh 2003;57:29-31. [PubMed]
- Shirabe K, Matsumata T, Shimada M, et al. A comparison of parenteral hyperalimentation and early enteral feeding regarding systemic immunity after major hepatic resection-the results of a randomized prospective study. Hepatogastroenterology 1997;44:205-209. [PubMed]
- Mochizuki H, Togo S, Tanaka K, et al. Early enteral nutrition after hepatectomy to prevent postoperative infection. Hepatogastroenterology 2000;47:1407-10. [PubMed]
- Richter B, Schmandra TC, Golling M, et al. Nutritional support after open liver resection: a systematic review. Dig Surg 2006;23:139-145. [PubMed]
- August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472-500. [PubMed]
- Okabayashi T, Nishimori I, Sugimoto T, et al. Effects of branched-chain amino acids: enriched nutrient support for patients undergoing liver resection for hepatocellular carcinoma. J Gastroenterol Hepatol 2008;23:1869-73. [PubMed]
- Plauth M, Cabré E, Riggio O, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr 2006;25:285-94. [PubMed]
- Buchman AL. Total parenteral nutrition: challenges and practice in the cirrhotic patient. Transplant Proc 2006;38:1659-63. [PubMed]
- Zaloga GP, Roberts P. Permissive underfeeding. New Horiz 1994;2:257-63. [PubMed]
- Patiño JF, de Pimiento SE, Vergara A, et al. Hypocaloric support in the critically ill. World J Surg 1999;23:553-9. [PubMed]
- McCowen KC, Friel C, Sternberg J, et al. Hypocaloric total parenteral nutrition: rffectiveness in prenention of hyperglycemia and infectious complications: a randomized clinical trial. Crit Care Med 2000;28:3606-11. [PubMed]
- Yilei Mao, Xin Lu, Xinting Sang, et al. Permissive underfeeding in post-operative patients: results of a prospective, randomized, controlled clinical trial. Chin J Gen Surg 2005;20:612-5.
- Hallas P. Glucose control in critically ill patients. N Engl J Med 2009;361:91. [PubMed]
- Barton RG. Nutrition support in critical illness. Nutr Clin Pract 1994;9:127-39. [PubMed]
- Kitchen P, Forbes A. Intravenous nutrition in critical illness. Curr Opin Gastroenterol 2001;17:150-3. [PubMed]
- Chambrier C, Lauverjat M, Bouletreau P. Structured triglyceride emulsions in parenteral nutrition. Nutr Clin Pract 2006;21:342-50. [PubMed]
- Wichmann MW, Thul P, Czarnetzki HD, et al. Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med 2007;35:700-6. [PubMed]
- Kohl M, Wedel T, Entenmann A, et al. Influence of different intravenous lipid emulsions on hepatobiliary dysfunction in a rabbit model. J Pediatr Gastroenterol Nutr 2007;44:237-44. [PubMed]
- Li NN, Zhou Y, Qin XP, et al. Does intravenous fish oil benefit patients post-surgery? A meta-analysis of randomised controlled trials. Clin Nutr 2014;33:226-39. [PubMed]
- Pscheidl E, Schywalsky M, Tschaikowsky K, et al. Fish oil-supplemented parenteral diets normalize splanchnic blood flow and improve killing of translocated bacteria in a low dose endotoxin rat model. Crit Care Med 2000;28:1489-96. [PubMed]
- Fürst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clin Nutr 2000;19:7-14. [PubMed]
- Mayer K, Seeger W. Fish oil in critical illness. Curr Opin Clin Nutr Metab Care 2008;11:121-7. [PubMed]
- Tian H, Yao X, Zeng R, et al. Safety and efficacy of a new parenteral lipid emulsion (SMOF) for surgical patients: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev 2013;71:815-21. [PubMed]
- Calder PC. Branched-chain amino acids and immunity. J Nutr 2006;136:288S-93S. [PubMed]
- Choudry HA, Pan M, Karinch AM, et al. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr 2006;136:314S-8S. [PubMed]
- Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr 2006;136:295S-8S. [PubMed]
- Holecek M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition 2010;26:482-90. [PubMed]
- Nakaya Y, Okita K, Suzuki K, et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 2007;23:113-20. [PubMed]
- Moriwaki H, Shiraki M, Fukushima H, et al. Long-term outcome of branched-chain amino acid treatment in patients with liver cirrhosis. Hepatol Res 2008;38:S102-6. [PubMed]
- Kobayashi M, Ikeda K, Arase Y, et al. Inhibitory effect of branched-chain amino acid granules on progression of compensated liver cirrhosis due to hepatitis C virus. J Gastroenterol 2008;43:63-70. [PubMed]
- Habu D, Nishiguchi S, Nakatani S, et al. Comparison of the effect of BCAA granules on between decompensated and compensated cirrhosis. Hepatogastroenterology 2009;56:1719-23. [PubMed]
- Meng WC, Leung KL, Ho RL, et al. Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Aust N Z J Surg 1999;69:811-5. [PubMed]
- Poon RT, Yu WC, Fan ST, et al. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Aliment Pharmacol Ther 2004;19:779-88. [PubMed]
- Takeshita S, Ichikawa T, Nakao K, et al. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res 2009;29:89-93. [PubMed]
- Ishikawa T, Michitaka I, Kamimura H, et al. Oral branched-chain amino acids administration improves impaired liver dysfunction after radiofrequency ablation therapy for hepatocellular carcinoma. Hepatogastroenterology 2009;56:1491-5. [PubMed]
- Kuroda H, Ushio A, Miyamoto Y, et al. Effects of branched-chain amino acid-enriched nutrient for patients with hepatocellular carcinoma following radiofrequency ablation: a one-year prospective trial. J Gastroenterol Hepatol 2010;25:1550-5. [PubMed]
- Okabayashi T, Iyoki M, Sugimoto T, et al. Oral supplementation with carbohydrate and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids 2011;40:1213-20. [PubMed]
- Togo S, Tanaka K, Morioka D, et al. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition 2005;21:480-6. [PubMed]
- Ishikawa Y, Yoshida H, Mamada Y, et al. Prospective randomized controlled study of short-term perioperative oral nutrition with branched chain amino acids in patients undergoing liver surgery. Hepatogastroenterology 2010;57:583-90. [PubMed]
- Allison SP, Lobo DN. Debate: albumin administration should not be avoided. Crit Care 2000;4:147-50. [PubMed]
- Giovannini I, Chiarla C, Giuliante F, et al. The relationship between albumin, other plasma proteins and variables, and age in the acute phase response after liver resection in man. Amino Acids 2006;31:463-9. [PubMed]
- Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247-56. [PubMed]
- Allison SP, Lobo DN. Debate: albumin administration should not be avoided. Crit Care 2000;4:147-150. [PubMed]
- Haynes GR, Navickis RJ, Wilkes MM. Albumin administration--what is the evidence of clinical benefit? A systematic review of randomized controlled trials. Eur J Anaesthesiol 2003;20:771-93. [PubMed]
- Uhing MR. The albumin controversy. Clin Perinatol 2004;31:475-88. [PubMed]
- Haynes GR, Navickis RJ, Wilkes MM. Albumin administration-what is the evidence of clinical benefit? A systematic review of randomized controlled trials. Eur J Anaesthesiol 2003;20:771-93. [PubMed]
- Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860-9; discussion 869-70. [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant heaptobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698-708. [PubMed]
- Vincent JL, Naviekis RJ, Wilkes MM. Morhidity in hospitalized patients receiving human albumin:a meta-analysis of randomized, controlled trial. Crit Care Med 2004;32:2029-38. [PubMed]
- Groeneveld AB, Navickis RJ, Wilkes MM. Update on the comparative safety of colloids: asyslematic review of clinical studies. Ann Surg 2011;253:470-83. [PubMed]
- Cochrane Injuries Grnup Albumin Reviewers. Human albumin administration in critically ill patients:systematic review of randnmised controlled trials. BMJ 1998;317:235-40. [PubMed]
- Davidson IJ. Renal impact of fluid managemenl with colloids: a comparative review. Eur J Anaesthesiol 2006;23:721-38. [PubMed]
- Barron ME, Wilkes MM, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg 2004;139:552-63. [PubMed]
- Reinhart K, Perner A, Sprung CL, et al. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med 2012;38:368-83. [PubMed]


