Multiple variants in UGT1A1 gene are factors to develop indirect hyper-bilirubinemia
Introduction
Hereditary abnormalities of uridine diphosphoglucuronate-glucuronosyltransferase 1A1 (UGT1A1) gene is a major cause of unconjugated hyper-bilirubinemia in Taiwan and worldwide (1-4). Patients with Gilbert’s syndrome are linked to a variation in the promoter of the UGT1A1: an additional TA dinucleotide in the TATA box resulting in a (TA)7TAA7 genotype (5,6). Our earlier study showed that there is a correlation between the increase of serum bilirubin level and the frequency of variants in the UGT1A1 gene in healthy Taiwanese adults; heterozygous variants in UGT1A1 are common in healthy Taiwanese adults without hyper-bilirubinemia (7). Also, multiple studies have reported that additional molecular defects in UGT1A1 are frequently identified in patients with Gilbert’s syndrome (8-10). Therefore, we investigated the role of variants in UGT1A1 gene on the pathogenesis of indirect hyper-bilirubinemia.
Patients and methods
We prospectively studied 97 consecutive documented patients (female: 21, male: 76; mean age ± SD: 39.0±11.9 years old) at the Cathay General Hospital, Taipei, Taiwan with total bilirubin levels ≥2.0 mg/dL (2.39±0.49 mg/dL) from 1998 to 2008. Indirect hyper-bilirubinemia was defined by having unconjugated bilirubin/total bilirubin >80% over a period greater than 4 months.
All patients had a normal mean corpuscular volume (MCV) with mean hemoglobin 15.3±1.2 mg/dL and normal reticulocyte counts. None of the patients had any medications within 8 weeks. Patients with viral hepatitis, abnormal serum alanine aminotransferase and aspartate aminotransferase values, alcoholism, other hemolytic diseases such as glucose-6-phosphate dehydrogenase (G6PD) deficiency and thalassemia were excluded.
Blood samples were collected in the morning, after overnight fasting for more than 8 hours under a regular dietary intake. Serum bilirubin levels were assayed using Autoanalyser (model 747, Hitachi Co., Tokyo, Japan).
Total genomic DNA was isolated from whole blood cells using a blood DNA isolation kit (Maxim Biotech Inc., San Francisco, CA, USA). The five exons of the UGT1A1 gene including the flanking intronic regions and promoter area were amplified using 15 natural or mutagenic primers by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method described in previous publication (11). The PCR-RFLP technique was used to genotype the promoter and the coding region of the UGT1A1 gene for the five known variants in Taiwan as our previous publication (9). The A(TA)6TAA to A(TA)7TAA variants in the promoter were classified as homozygous 6/6, homozygous 7/7, and heterozygous 6/7. The five known variants at the coding region include nucleotide (nt)-211 G to A (G71R), nt-686 C to A (P229Q) at exon 1, nt-1,091 C to T (P364L) at exon 4 and nt-1,456 T to G (Y486D) at exon 5.
Erythrocyte G6PD activity was quantitated using the enzyme-coupled method described in previous publication (12). MCV was measured by using Sysmex XE 2100 (Sysmex Corp., Tokyo, Japan). Subjects with MCV ≤80 fL were assessed for thalassemia by using a polymerase chain reaction method described in previous publication (13). Briefly, the occurrence of SEA deletion for α-thalassemia and codon 41/42 (-TTCT), intervening sequences (IVS) II654 (C>T), -28 (A>G), and codon 17 (A>T) in PCR-RFLP for β-thalassemia.
All subjects provided written informed consents. The study protocol was reviewed and approved by the Institutional Review Board under the guidelines of the World Medical Associations Declaration of Helsinki as revised by the World Medical Organization, Edinburgh 2000. Statistical analyses were performed using appropriate commercially-available software (Microsoft Excel 2007, MapInfo Co., Tory, NJ; and SAS software version 9.2, SAS Institute, Cary, NC).
Results
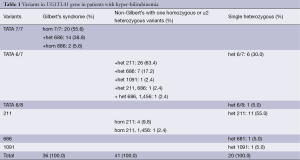
Our data showed that 79 patients (81.4%) had variants in the TATA box of promoter (Table 1). Thirty-six patients (45.6%) of them had the Gilbert’s syndrome with the 7/7 genotype; fourteen of the 36 patients also carried heterozygous variant at nt-686 and two patients carried homozygous variant at nt-686. On the other hand, one patient carried a unique heterozygous 6/8 genotype and 42 patients (53.2%) had the heterozygous 6/7 genotype. Among the 42 heterozygous 6/7 genotype patients, 36 patients were found to have additional variants in UGT1A1 (6/7+ nt-211: 26, 6/7+ nt-686: 7, 6/7+ nt-1,091: 1, 6/7+ nt-211+ nt-686: 1, 6/7+ nt-686+ nt-1,456: 1). In the remaining 18 patients (18.6%) without variants in the promoter, four of them had the homozygous nt-211 mutation, one had the homozygous nt-211 and heterozygous nt-1,456, 11 had the heterozygous nt-211 variant, one patient had the heterozygous nt-686 variant, and one patient had the heterozygous nt-1,091 variant.
Full table
Among the 97 patients, 43 patients (44.3%) carried the nt-211 variant. Only five of them had a homozygous nt-211 variant; one of the five had also carried a homozygous nt-1,456 variant. Of the 38 patients with heterozygous nt-211 variant, 11 had no other detectable molecular defect, 26 patients had the heterozygous 6/7 genotype at the promoter, and one patient had the heterozygous 6/7 genotype and also carries a heterozygous nt-686 variant.
Twenty-six patients (26.8%) had variant at nt-686. Two patients had homozygous nt-688 variant, and both patients are found to carry the homozygous 7/7 genotype. For the remaining 24 patients with nt-688 heterozygous variant, one patient had no other detectable molecular defect, 14 patients carried the homozygous 7/7 genotype, seven patients had the heterozygous 6/7 genotype, one patient had the heterozygous 6/7 genotype and a heterozygous nt-211 variant, and one patient had the heterozygous 6/7 genotype and a heterozygous nt-1,456 variant.
Two patients (2.1%) had the nt-1091 heterozygous variants. One patient had also the heterozygous 6/7 genotype and the other carries no other detectable molecular defect.
Two patients (2.1%) had the nt-1,456 heterozygous variants. One patient had the heterozygous 6/7 genotype and a heterozygous nt-686 variants, and the other patient had a homozygous nt-211 variants.
Forty-one (67.2%) of the 61 non-Gilbert’s patients had one homogenous variants or two or more heterozygous variants in UGT1A1. Five of them carried homozygous nt-211 variants, and 36 patients had two or more heterozygous variants. Only eight (19.5%) of these 41 non-Gilbert’s patients had serum total bilirubin levels above 2.5 mg/dL.
Only 20 patients (20.6%) were found to have one single heterozygous variant in coding region or promoter; 6 patients had the 6/7 genotype, one patient was with the 6/8 genotype, 11 patients had the nt-211 variant, one patient had the nt-686 variant, and one patient had the nt-1,091 variant. Only 4 of the 20 patients (20%) had serum total bilirubin levels above 2.5 mg/dL.
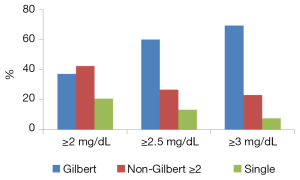
On the contrary, 20 of the 36 patients (55.6%) with Gilbert’ syndrome had serum total bilirubin levels above 2.5 mg/dL. The percentage of Gilbert’s syndrome genotype in all patients was 37.1%; however, in the 30 patients with serum total bilirubin above 2.5 mg/dL, 60.0% (Chi-square, χ2=9.75, P=0.007) had the Gilbert’s syndrome genotype; and in the 13 patients with serum total bilirubin above 3.0 mg/dL, 69.2% (χ2=6.70, P=0.035) had the Gilbert’s syndrome genotype (Figure 1). Only 23.9% of 65 patients with serum total bilirubin level <2.5 mg/dL carry the same genotype (χ2=11.87, P=0.0006). We did not find correlation between the number of variants in UGT1A1 and serum total bilirubin levels (correlation coefficient, r=0.2).
Discussions
Taiwan is a region with high prevalence of viral hepatitis and hepatocellular carcinoma (14,15). Recent economic prosperity has led to increased alcohol consumption, which results in increasing cases of alcoholic liver disease (16,17). Moreover, patients with UGT1A1 abnormalities may develop drug interactions and cholelithiasis with hyper-bilirubinemia (18-21). Our previous study showed that over 50% of healthy Taiwanese adults carry at least one heterozygous variant in the UGT1A1 gene (7). The differential diagnosis of hyper-bilirubinemia is therefore important in this region due to the possible coexisting viral hepatitis, liver malignancies, alcoholism, drug and cholelithiasis (22-24).
Our data show that most patients with hyper-bilirubinemia had variants in the promoter region of UGT1A1; half of them were confirmed to have the Gilbert’s syndrome’s homozygous 7/7 genotype. Our findings are consistent with previous reports that Gilbert syndrome is the most common inherited disorder of bilirubin glucuronidation (25,26). Moreover, two-fifth of the patients with Gilbert’s syndrome also carries heter- or homo-zygous variants at nt-686. Different from Western population, variants at nt-211 are common in Taiwan and Asian countries (27). Our study shows variants other than nt-211 in the coding region of UGT1A1 gene as a risk factor of hyperbilirubinemia are not uncommon. Our findings are consistent with earlier reports that polymorphism in the UGT1A1 gene are common in the Taiwanese population (23,28).
Two-fifth of the patients carried one or more homozygous variants; most of them had Gilbert’s syndrome’s 7/7 genotype and half of them had serum total bilirubin level above 2.5 mg/dL. Our data also showed that most patients with serum total bilirubin greater than 2.5 mg/dL carried the Gilbert’s syndrome genotype. On the contrary, the five non-Gilbert’s patients with homozygous nt-211 mutation had serum total bilirubin level below 2.5 mg/dL.
It is interesting to note that that the two-fifth of patients without the Gilbert’s syndrome genotype had one homogenous variant or two or more heterozygous variants in UGT1A1; most of them had serum total bilirubin level below 2.5 mg/dL. In one rare case, a patient has a 6/8 genotype and had serum total bilirubin level at 2.2 mg/dL, consistent with an earlier report that there is a reduced transcription activity of UGT1A1 gene in 6/8 genotype (29). We did not find correlation between the number of variants in UGT1A1 gene and serum total bilirubin levels.
In this study, most non-Gilbert’s patients with hyper-bilirubinemia carry one homo-zygous variant or two or more hetero-zygous variants in UGT1A1. We hypothesize that non-Gilbert patients with one homo-zygous variant or two or more hetero-zygous variants in UGT1A1 gene develop a mild form of hyper-bilirubinemia. One-fifth of the indirect hyper-bilirubinemia patients had only one single heterozygous variant. There may be other unidentified driver for indirect hyper-bilirubinemia.
The present study has some limitations. Our case number is limited; further studies with much larger number of enrolled patients are necessary to confirm the role of multiple variants as factors for developing indirect hyper-bilirubinemia. We excluded cases with coexisting hematological disorders such as G6PD deficiency and thalassemia (30,31). Furthermore, we also excluded patients with other liver diseases such as chronic viral hepatitis B, which accounts for 15% of the population aged more than 30 years old in Taiwan (32,33). Thus the effect of UGT1A1 variants might have been underestimated in this study.
In conclusion, our data suggest that the percentage of Gilbert’s syndrome increased in patients with bilirubin above 2.5 mg/dL. Most patients without the Gilbert’s syndrome genotype had one homogenous variants or two or more heterozygous variants in UGT1A1; most of them had serum total bilirubin level below 2.5 mg/dL. Further studies are necessary to confirm the role of multiple variants in UGT1A1 gene as factors for indirect hyper-bilirubinemia.
Acknowledgements
The authors thank Zinger Yang, MSc. and Szu-Chieh Fu, MSc. for their assistance in the preparation of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Muraca M, Fevery J, Blanckaert N. Relationships between serum bilirubins and production and conjugation of bilirubin. Studies in Gilbert’s syndrome, Crigler-Najjar disease, hemolytic disorders, and rat models. Gastroenterology 1987;92:309-17. [PubMed]
- Fretzayas A, Moustaki M, Liapi O, et al. Gilbert syndrome. Eur J Pediatr 2012;171:11-5. [PubMed]
- Raijmakers MT, Jansen PL, Steegers EA, et al. Association of human liver bilirubin UDP-glucuronyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. J Hepatol 2000;33:348-51. [PubMed]
- Huang CS, Chang PF, Huang MJ, et al. Relationship between bilirubin UDP-glucuronosyl transferase 1A1 gene and neonatal hyperbilirubinemia. Pediatr Res 2002;52:601-5. [PubMed]
- Monaghan G, Ryan M, Seddon R, et al. Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 1996;347:578-81. [PubMed]
- Bosma PJ, Chowdhury JR, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 1995;333:1171-5. [PubMed]
- Huang CS, Luo GA, Huang ML, et al. Variations of the bilirubin uridine-diphosphoglucuronosyl transferase 1A1 gene in healthy Taiwanese. Pharmacogenetics 2000;10:539-44. [PubMed]
- Huang MJ, Yang YC, Yang SS, et al. Coinheritance of variant UDP-glucuronosyl transferase 1A1 gene and glucose-6-phosphate dehydrogenase deficiency in adults with hyperbilirubinemia. Pharmacogenetics 2002;12:663-6. [PubMed]
- Huang CS, Chang PF, Huang MJ, et al. Glucose-6-phosphate dehydrogenase deficiency, the UDP-glucuronosyl transferase 1A1 gene, and neonatal hyperbilirubinemia. Gastroenterology 2002;123:127-33. [PubMed]
- Galanello R, Cipollina MD, Carboni G, et al. Hyperbilirubinemia, glucose-6-phosphate-dehydrogenase deficiency and Gilbert’s syndrome. Eur J Pediatr 1999;158:914-6. [PubMed]
- Huang MJ, Kua KE, Teng HC, et al. Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res 2004;56:682-9. [PubMed]
- Huang CS, Hung KL, Huang MJ, et al. Neonatal jaundice and molecular mutations in glucose-6-phosphate dehydrogenase deficient newborn infants. Am J Hematol 1996;51:19-25. [PubMed]
- Lin HJ, Shih MC, Peng CT, et al. Hematological features and molecular lesions of hemoglobin gene disorders in Taiwanese patients. Int J Lab Hematol 2010;32:1-7. [PubMed]
- Chu CM, Karayiannis P, Fowler MJ, et al. Natural history of chronic hepatitis B virus infection in Taiwan: studies of hepatitis B virus DNA in serum. Hepatology 1985;5:431-4. [PubMed]
- Kao JH, Chen PJ, Lai MY, et al. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 2000;118:554-9. [PubMed]
- Lin CW, Lin CC, Mo LR, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol 2013;58:730-5. [PubMed]
- Lin CW, Chen YS, Lai CH, et al. Esophagogastric varices predict mortality in hospitalized patients with alcoholic liver disease in Taiwan. Hepatogastroenterology 2010;57:305-8. [PubMed]
- de Morais SM, Uetrecht JP, Wells PG. Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert’s syndrome. Gastroenterology 1992;102:577-86. [PubMed]
- Deterding K, Grüngreiff K, Lankisch TO, et al. Gilbert’s syndrome and antiviral therapy of hepatitis C. Ann Hepatol 2009;8:246-50. [PubMed]
- Carulli N, Ponz de Leon M, et al. Alteration of drug metabolism in Gilbert’s syndrome. Gut 1976;17:581-7. [PubMed]
- del Giudice EM, Perrotta S, Nobili B, et al. Coinheritance of Gilbert syndrome increases the risk for developing gallstones in patients with hereditary spherocytosis. Blood 1999;94:2259-62. [PubMed]
- Huang CS, Huang MJ, Lin MS, et al. Genetic factors related to unconjugated hyperbilirubinemia amongst adults. Pharmacogenet Genomics 2005;15:43-50. [PubMed]
- Linn S, Schoenbaum SC, Monson RR, et al. Epidemiology of neonatal hyperbilirubinemia. Pediatrics 1985;75:770-4. [PubMed]
- Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology 2008;135:111-21. [PubMed]
- Borlak J, Thum T, Landt O, et al. Molecular diagnosis of a familial nonhemolytic hyperbilirubinemia (Gilbert’s syndrome) in healthy subjects. Hepatology 2000;32:792-5. [PubMed]
- Sieg A, Arab L, Schlierf G, et al. Dtsch Med Wochenschr 1987;112:1206-8. [PubMed]
- Sai K, Saeki M, Saito Y, et al. UGT1A1 haplotypes associated with reduced glucuronidation and increased serum bilirubin in irinotecan-administered Japanese patients with cancer. Clin Pharmacol Ther 2004;75:501-15. [PubMed]
- Huang MJ, Yang SS, Lin MS, et al. Polymorphisms of uridine-diphosphoglucuronosyltransferase 1A7 gene in Taiwan Chinese. World J Gastroenterol 2005;11:797-802. [PubMed]
- Iolascon A, Faienza MF, Centra M, et al. (TA)8 allele in the UGT1A1 gene promoter of a Caucasian with Gilbert’s syndrome. Haematologica 1999;84:106-9. [PubMed]
- Sampietro M, Lupica L, Perrero L, et al. The expression of uridine diphosphate glucuronosyltransferase gene is a major determinant of bilirubin level in heterozygous beta-thalassaemia and in glucose-6-phosphate dehydrogenase deficiency. Br J Haematol 1997;99:437-9. [PubMed]
- Iolascon A, Faienza MF, Giordani L, et al. Bilirubin levels in the acute hemolytic crisis of G6PD deficiency are related to Gilbert’s syndrome. Eur J Haematol 1999;62:307-10. [PubMed]
- Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis 2002;2:395-403. [PubMed]
- Ni YH, Huang LM, Chang MH, et al. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology 2007;132:1287-93. [PubMed]


