Post-hepatectomy liver failure
Introduction
Hepatic resections are among some of the most complex operative interventions performed, and are fraught with risk and the potential for complications. Mortality rates after major hepatic resection have been reported to be as high as 30% (1,2) with post-hepatectomy liver failure (PHLF) representing the major source of morbidity and mortality after liver resection. Despite great improvements in outcomes after major liver resection due to refinements in operative technique and advances in critical care, PHLF remains one of the most serious complications of major liver resection, and occurs in up to 10% of cases (3,4). Several studies report a lower rate of PHLF in East Asian countries (1-2%), but when present, PHLF represents a significant source of morbidity and mortality (5).
Definition
The definition of PHLF has varied widely among groups, making comparison of rates between studies challenging. Numerous definitions of PHLF exist in the literature, with variations by country and between hospitals within the same country. Many definitions include complicated formulas or obscure laboratory tests, such as hepaplastin or hyaluronic acid levels, limiting their utility (6). The Model for End-Stage Liver Disease (MELD) score is one such definition that is widely used. The MELD score is calculated using serum creatinine, INR, and bilirubin, but requires a complex mathematical formula computation (7). The ‘50-50 criterion’ (PT <50% and bilirubin >50 µmL/L) have also been proposed as a simple definition for PHLF (8). However, this definition does not account for any clinical parameters, and relies only on two laboratory values. In 2011, the International Study Group of Liver Surgery (ISGLS) proposed a standardized definition and severity of grading of PHLF. After evaluating more than 50 studies on PHLF after hepatic resection, the consensus conference committee defined PHLF as “a post-operatively acquired deterioration in the ability of the liver to maintain its synthetic, excretory, and detoxifying functions, which are characterized by an increased INR and concomitant hyperbilirubinemia on or after postoperative day 5” (2). While other definitions of PHLF utilizing biochemical or clinical parameters are used by some centers, the ease with which the ISGLS definition can be calculated and used for comparison renders it the definition that ought to be standardized and used.
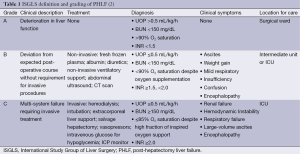
While PHLF is the most feared complication, the severity of its clinical manifestation ranges from temporary hepatic insufficiency to fulminant hepatic failure. The ISGLS group advocated a simple grading system of PHLF, in which laboratory values, clinical symptoms, and need for increasingly invasive treatments define severity of PHLF. The mildest grade of PHLF, grade A, represents a minor, temporary deterioration in liver function that does not require invasive treatment or transfer to the intensive care unit. The most severe, grade C, is characterized by severe liver failure with multisystem failure and the requirement for management of multi-system failure in the intensive care unit (2) (Table 1). The peri-operative mortality of patients with grades A, B, and C PHLF as determined by this grading schema is 0%, 12% and 54%, respectively (9).
Predictive factors
Patient factors
Various patient-related factors are associated with increased risk of PHLF (Table 2). Operative mortality in patients with diabetes undergoing curative-intent hepatic resection for treatment of colorectal metastases has been shown to be higher than comparable patients without diabetes mellitus (6). In that series, operative mortality was 8% in diabetics compared to 2% in non-diabetics (P<0.02). Furthermore, 80% of peri-operative deaths in diabetic patients were secondary to PHLF. Excess mortality seen in diabetic patients undergoing major hepatic resection is likely multi-factorial, with alterations in liver metabolism, decreased immune function, and hepatic steatosis contributing to post-operative liver dysfunction (10).

Full table
Chemotherapy-associated steatohepatitis (CASH) is an increasing challenge in the era of novel chemotherapeutic and biologic agents. Many commonly-used chemotherapy agents cause damage to hepatocytes, including 5-fluorouracil, irinotecan, oxaliplatin, cituximab, and bevacizumab (11-14). Additionally, pre-operative malnutrition or renal insufficiency, hyperbilirubinemia, thrombocytopenia, presence of co-morbidities (lung disease), and advanced age are associated with increased risk of PHLF (15-18).
Surgical factors
In addition to patient-specific factors, the performance of the surgical procedure itself influences risk of PHLF. Factors associated with increased risk are shown in Table 2 and include operative estimated blood loss >1,200 mL (19,20), intra-operative transfusion requirement, need for vena caval or other vascular resection (21), operative time >240 minutes (13), resection of >50% of liver volume, major hepatectomy including right lobe (22), and skeletonization of the hepatoduodenal ligament in cases of biliary malignancy (23). In patients for whom <25% of the pre-operative liver volume is left post-resection, the risk of PHLF is 3 times that of patients with ≥25% of liver volume remaining (24).
Post-operative factors
Issues of post-operative management influence the risk of PHLF, with post-operative hemorrhage (15) and occurrence of intra-abdominal infection (16) conferring increased risk (Table 2).
Pre-operative risk assessment
Given the high mortality rate associated with PHLF, there has been great interest in techniques to pre-operatively identify patients at high risk for hepatic dysfunction or failure. CT-based volumetric analysis is an effective tool that utilizes helical CT scans to assess the volume of resection by semi-automated contouring of the liver. A study by Shoup et al. utilized this technique to show that the percentage of remaining liver was closely correlated with increasing prothrombin time (>18 seconds) and bilirubin level (>3 mg/dL) (24). In their analysis, 90% of patients undergoing trisegmentectomy with ≤25% of liver remaining developed hepatic dysfunction, compared to none of the patients who had >25% of liver remaining after the same operation (24). Furthermore, the percentage of remaining liver, as determined by volumetric analysis, was more specific in predicting PHLF than the anatomic extent of resection (24).
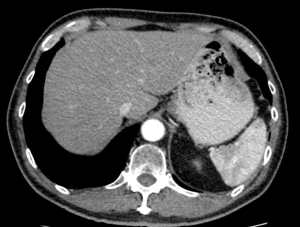
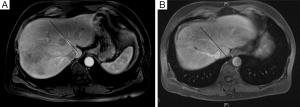
Careful evaluation of pre-operative CT scan imaging should focus on liver attenuation. Liver attenuation that is lower than that observed in the spleen indicates fatty infiltration indicative of steatohepatitis (11,24,25) (Figure 1). Similarly, splenomegaly, varices, ascites, or consumptive thrombocytopenia should prompt the clinician to suspect underlying cirrhosis (11) (Figure 2A,B).
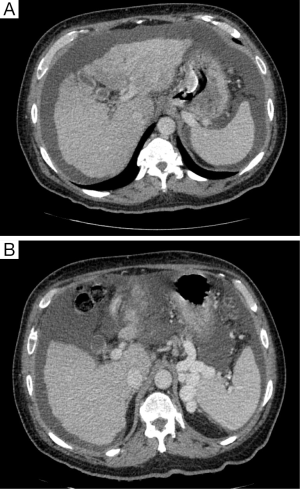
Although ultrasound and 3-dimensional ultrasound has been advocated by some as a means by which to assess the pre-operative volume of the liver, CT or MRI provide more objective data that is less subject to operator-error. Both CT and MRI show excellent accuracy and precise quantification of hepatic volume (26-28), and are particularly useful in estimating the future liver remnant (FLR) (29).
Numerous methods have been developed for calculating liver volume, using either CT or MRI images. The first technique involved manual tracing of the outline of the liver (30), but has been criticized its time-intensity. Most recently, automatic or semi-automatic techniques have been developed that utilize mathematical formulas to measure liver volumes obtained from CT scan images, utilizing commercially-available software programming. These software-based programs have been shown to correlate well with manual volume estimation, but are performed in a fraction of the time (31).
Although pre-operative estimation of functional liver volume after resection remains the most advanced method for estimating hepatic functional reserve, newer techniques, such as indocyanine green (ICG) clearance and ICG retention rate (ICG R15) have been reported. Under normal conditions, nearly all ICG administered is cleared by the liver. Because the ICG reflects intra-hepatic blood flow, it has long been used to assess liver functional reserve in patients with cirrhosis (32). Only recently, however, have investigations begun into the application of ICG and ICG R15 to estimating functional hepatic reserve after resection of normal livers in the setting of malignancy. In this method, ICG elimination is measured by pulse spectrophotometry (32), and the indocyanine green plasma disappearance rate (ICG PDR) is determined. The study by de Liguori Carino and colleagues reported that when the pre-operative ICG PDR was less than 17.6%/min and the pre-operative serum bilirubin was >17 µmol/L, the positive predictive value for post-operative liver dysfunction was 75%, and the negative predictive value was 90% (32). While additional study is needed, this method appears to be a non-invasive tool for prediction of PHLF.
There is increasing interest in the use of 99mTc-diethylenetriamine-pentaacetic acid-galactosyl human serum albumin (GSA) scintigraphy for the pre-operative evaluation of cirrhotic patients. In this technique, the molecule is taken up by the liver, reflecting the volume of functional liver (33). Uptake corresponds to bilirubin level, INR, and ICG clearance (33). In 9-20% of patients, the severity of liver disease is underestimated by ICG clearance testing, and better represented by GSA scintigraphy. This may be due to the fact that GSA scintigraphy is unaffected by hyperbilirubinemia (33). Use of GSA scintigraphy pre-operatively allows for highly accurate estimation of FLR (33).
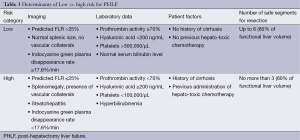
Beyond imaging, a number of laboratory parameters have been shown to correlate with risk of PHLF, including prothrombin activity <70% and hyaluronic acid level ≥200 ng/mL. When elevated pre-operatively, these values portend greater risk of PHLF (34), and can be used as indications for or against major hepatectomy (Table 3).
Full table
Prevention
Treatment of PHLF hinges first on its prevention. In patients identified as high-risk by preoperative evaluation of underlying patient factors, presence of cirrhosis, pre-operative laboratory values, volume of liver to be resected, or estimated functional liver volume after resection, consideration should be given to techniques to minimize the risk of PHLF. One such technique is portal vein embolization (PVE), which manipulates portal blood flow, by embolizing portal branches in the liver to be resected, directing blood flow to the intended remnant liver, and thereby inducing hypertrophy of the remnant liver before major hepatectomy (35). By increasing the volume of the intended remnant liver, the risk for PHLF is decreased, even after extended liver resection. Furthermore, pre-operative PVE minimizes intra-operative hepatocyte injury that would otherwise be caused by the abrupt increase in portal venous pressure at the time of resection (35). Current guidelines recommend PVE for patients with underlying cirrhosis and an anticipated FLR of ≤40%, or patients with normal liver function and intended FLR of <20% (35). This procedure can be performed with minimal morbidity and mortality, and allows for improved safety of extended hepatectomies (36,37). Even when concurrent neoadjuvant chemotherapy is administered, sufficient hepatic hypertrophy occurs after PVE to allow for major liver resection (38). CT volumetry should be performed 3-4 weeks after PVE to assess the degree of hypertrophy (35). A degree of hypertrophy >5% is associated with improved patient outcomes (39) (Figure 3A,B).
Access to the portal system for PVE can be performed via transhepatic contralateral or transhepatic ipsilateral approach. The transhepatic contralateral approach accesses the portal system through the intended FLR, and is technically easier than an ipsilateral approach, but risks injury to the FLR. Additionally, access to segment 4 for embolization is technically difficult when performed from a contralateral approach (35). While the transhepatic ipsilateral approach spares the FLR from potential injury, acute angulations of the portal branches may render this approach too technically difficult to be feasible (35). If an extended right hepatectomy is planned, segment 4 could be embolized first to minimize risk of dislodgement of embolic substances to the left liver during manipulation of the catheter (35).
Because PVE is not always technically feasible and some patients may experience disease progression during the waiting time between PVE and surgery, the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure has been advocated by some, particularly for patients requiring trisectionectomy for bilateral liver metastases, or intrahepatic cholangiocarcinoma. In this procedure, blood supply to segments 4-8 is diminished by right portal vein branch ligation, combined with parenchymal transaction along the falciform ligament (40). This technique has shown a 74% increase in the volume of the FLR, but with high postoperative morbidity (68%) and mortality (12%) (41). Although there have been promising results in small series, with rapid liver hypertrophy and enlargement of the FLR, this technique requires additional study to refine its indications and place in the repertoire of techniques for minimizing the risk of PHLF (42).
Beyond pre-operative techniques to enlarge the FLR, fastidious intra-operative technique and excellent post-operative management contribute greatly to minimizing the risk of PHLF (Table 4). In cases of very heavy disease burden in the liver, when resection of all lesions would result in an FLR too small to avoid PHLF, a combination of resection and ablation may be used to minimize the amount of liver resected. Additionally, wedge resections with minimal tumor-free margins may be used to treat multi-focal disease, leaving sufficient liver intact to avoid PHLF.

Full table
Identification and management
When present, PHLF is manifest by progressive multi-system organ failure, including renal insufficiency, encephalopathy, need for ventilator support, and need for pressor support. As hepatic function worsens, patients develop persistent hyperbilirubinemia and coagulopathy (43). The development of coagulopathy is a particularly poor prognostic indicator (20). Daily measurement of serum C-reactive protein (CRP) may help with the early identification of patients who are developing hepatic insufficiency after hepatectomy. A study by Rahman and colleagues showed that patients who developed PHLF had a lower CRP level on post-operative day 1 than patients who did not develop PHLF. A serum CRP <32 g/dL was an independent predictor of PHLF in multivariate regression analysis (44). Other tools for predicting PHLF include the ‘50-50 criteria’, MELD system, and Acute Physiology and Chronic Health Evaluation (APACHE) III. While the MELD system has a sensitivity of 55% for morbidity and 71% for mortality, the ISGLS criteria for PHLF perform particularly well in assessing the risk of increased mortality after hepatectomy (45). The 50-50 criterion allows for early detection of PHLF, but is not a marker for increased morbidity after liver resection (45). The APACHE III score predicts mortality after hepatectomy, but has only been validated in patients with cholangiocellular carcinoma (46).
The most effective treatment for PHLF is liver transplantation, but this is typically reserved for patients who have failed all other supportive therapies (47). Initial treatment of PHLF includes supportive care of failing systems, including intubation, pressors, or dialysis. Treatment includes infusion of albumin, fibrinogen, fresh frozen plasma, blood transfusion, and initiation of nutritional supplementation (20).
Intra-hepatic cholestasis is a type of PHLF that warrants particular mention. It is characterized by a continued increase in serum bilirubin, in the absence of biliary obstruction, with preservation of the synthetic function of the liver (48). Biopsy confirming this entity should be obtained at 2 weeks post-operatively, if the diagnosis remains uncertain. Although the course is protracted, PHLF nearly always occurs, with mortality rates approaching 90% despite best supportive care.
Conclusions
PHLF remains a severe complication of hepatic resection, occurring in approximately 8% of patients undergoing major hepatectomy (49). It ranges from mild hepatic insufficiency, characterized by transient hyperbilirubinemia that does not alter the expected post-operative course, to liver failure resulting in multi-system failure requiring invasive treatment in an intensive care unit. Multiple factors increase the risk of PHLF, including obesity, diabetes, neoadjuvant treatment with chemotherapy, underlying cirrhosis, increased age, male gender, need for extended liver resection, and long operation with high intra-operative EBL. Risk of PHLF can be minimized by accurate pre-operative assessment of the FLR to be left after resection, and the induction of hypertrophy of the liver remnant via PVE if the expected FLR is <20% in a person with a normal liver, <30% in a patient with steatosis, or <40% in a cirrhotic patient (50). Early recognition and initiation of supportive care is crucial to improving patient survival in the setting of PHLF. Despite great improvements in morbidity and mortality, liver surgery continues to demand excellent clinical judgement in selecting patients for surgery. Appropriate choice of pre-operative techniques to improve the functional liver remnant (FLR), fastidious surgical technique, and excellent post-operative management are essential to optimize patient outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ong GB, Lee NW. Hepatic resection. Br J Surg 1975;62:421-30. [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [PubMed]
- Paugam-Burtz C, Janny S, Delefosse D, et al. Prospective validation of the "fifty-fifty" criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg 2009;249:124-8. [PubMed]
- Jaeck D, Bachellier P, Oussoultzoglou E, et al. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl 2004;10:S58-63. [PubMed]
- Ren Z, Xu Y, Zhu S. Indocyanine green retention test avoiding liver failure after hepatectomy for hepatolithiasis. Hepatogastroenterology 2012;59:782-4. [PubMed]
- Eguchi H, Umeshita K, Sakon M, et al. Presence of active hepatitis associated with liver cirrhosis is a risk factor for mortality caused by posthepatectomy liver failure. Dig Dis Sci 2000;45:1383-8. [PubMed]
- Yoo HY, Edwin D, Thuluvath PJ. Relationship of the model for end-stage liver disease (MELD) scale to hepatic encephalopathy, as defined by electroencephalography and neuropsychometric testing, and ascites. Am J Gastroenterol 2003;98:1395-9. [PubMed]
- Balzan S, Belghiti J, Farges O, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824-8, discussion 828-9. [PubMed]
- Reissfelder C, Rahbari NN, Koch M, et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg 2011;98:836-44. [PubMed]
- Little SA, Jarnagin WR, DeMatteo RP, et al. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg 2002;6:88-94. [PubMed]
- Fong Y, Bentrem DJ. CASH (Chemotherapy-Associated Steatohepatitis) costs. Ann Surg 2006;243:8-9. [PubMed]
- Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006;243:1-7. [PubMed]
- Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg 2005;200:845-53. [PubMed]
- Peppercorn PD, Reznek RH, Wilson P, et al. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer 1998;77:2008-11. [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. [PubMed]
- Tzeng CW, Cooper AB, Vauthey JN, et al. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford) 2014;16:459-68. [PubMed]
- Golse N, Bucur PO, Adam R, et al. New paradigms in post-hepatectomy liver failure. J Gastrointest Surg 2013;17:593-605. [PubMed]
- Aloia TA, Fahy BN, Fischer CP, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510-5. [PubMed]
- Tomuş C, Iancu C, Bălă O, et al. Liver resection for benign hepatic lesion: mortality, morbidity and risk factors for postoperative complications. Chirurgia (Bucur) 2009;104:275-80. [PubMed]
- Jin S, Fu Q, Wuyun G, et al. Management of post-hepatectomy complications. World J Gastroenterol 2013;19:7983-91. [PubMed]
- Melendez J, Ferri E, Zwillman M, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg 2001;192:47-53. [PubMed]
- Nanashima A, Yamaguchi H, Shibasaki S, et al. Comparative analysis of postoperative morbidity according to type and extent of hepatectomy. Hepatogastroenterology 2005;52:844-8. [PubMed]
- Fujii Y, Shimada H, Endo I, et al. Risk factors of posthepatectomy liver failure after portal vein embolization. J Hepatobiliary Pancreat Surg 2003;10:226-32. [PubMed]
- Shoup M, Gonen M, D’Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7:325-30. [PubMed]
- Panicek DM, Giess CS, Schwartz LH. Qualitative assessment of liver for fatty infiltration on contrast-enhanced CT: is muscle a better standard of reference than spleen? J Comput Assist Tomogr 1997;21:699-705. [PubMed]
- D’Onofrio M, De Robertis R, Demozzi E, et al. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J Radiol 2014;6:62-71. [PubMed]
- Tu R, Xia LP, Yu AL, et al. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol 2007;13:3956-61. [PubMed]
- Torzilli G, Montorsi M, Del Fabbro D, et al. Ultrasonographically guided surgical approach to liver tumours involving the hepatic veins close to the caval confluence. Br J Surg 2006;93:1238-46. [PubMed]
- Ulla M, Ardiles V, Levy-Yeyati E, et al. New surgical strategy to induce liver hypertrophy: role of MDCT-volumetry to monitor and predict liver growth. Hepatogastroenterology 2013;60:337-42. [PubMed]
- Soyer P, Roche A, Elias D, et al. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology 1992;184:695-7. [PubMed]
- Suzuki K, Epstein ML, Kohlbrenner R, et al. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am J Roentgenol 2011;197:W706-12. [PubMed]
- de Liguori Carino N, O’Reilly DA, Dajani K, et al. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol 2009;35:957-62. [PubMed]
- Hoekstra LT, de Graaf W, Nibourg GA, et al. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg 2013;257:27-36. [PubMed]
- Nanashima A, Tobinaga S, Abo T, et al. Reducing the incidence of post-hepatectomy hepatic complications by preoperatively applying parameters predictive of liver function. J Hepatobiliary Pancreat Sci 2010;17:871-8. [PubMed]
- Thakrar PD, Madoff DC. Preoperative portal vein embolization: an approach to improve the safety of major hepatic resection. Semin Roentgenol 2011;46:142-53. [PubMed]
- Shindoh J, Tzeng CW, Aloia TA, et al. Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg 2014;18:45-51. [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [PubMed]
- Covey AM, Brown KT, Jarnagin WR, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg 2008;247:451-5. [PubMed]
- Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94. [PubMed]
- Li J, Girotti P, Königsrainer I, et al. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg 2013;17:956-61. [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [PubMed]
- Vennarecci G, Laurenzi A, Levi Sandri GB, et al. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol 2014;40:982-8. [PubMed]
- Roberts KJ, Bharathy KG, Lodge JP. Kinetics of liver function tests after a hepatectomy for colorectal liver metastases predict post-operative liver failure as defined by the International Study Group for Liver Surgery. HPB (Oxford) 2013;15:345-51. [PubMed]
- Rahman SH, Evans J, Toogood GJ, et al. Prognostic utility of postoperative C-reactive protein for posthepatectomy liver failure. Arch Surg 2008;143:247-53; discussion 253. [PubMed]
- Rahbari NN, Reissfelder C, Koch M, et al. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol 2011;18:3640-9. [PubMed]
- Hamahata N, Nagino M, Nimura Y. APACHE III, unlike APACHE II, predicts posthepatectomy mortality in patients with biliary tract carcinoma. Acute Physiology and Chronic Health Evaluation. Crit Care Med 1998;26:1671-6. [PubMed]
- Chan SC, Sharr WW, Chan AC, et al. Rescue Living-donor Liver Transplantation for Liver Failure Following Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2013;2:332-7. [PubMed]
- Chiarla C, Giovannini I, Giuliante F, et al. Plasma bilirubin correlations in non-obstructive cholestasis after partial hepatectomy. Clin Chem Lab Med 2008;46:1598-601. [PubMed]
- Vibert E, Pittau G, Gelli M, et al. Actual incidence and long-term consequences of posthepatectomy liver failure after hepatectomy for colorectal liver metastases. Surgery 2014;155:94-105. [PubMed]
- Asencio JM, García Sabrido JL, Olmedilla L. How to expand the safe limits in hepatic resections? J Hepatobiliary Pancreat Sci 2014;21:399-404. [PubMed]