Complications after percutaneous ablation of liver tumors: a systematic review
Introduction
Hepatocellular carcinoma (HCC) and colorectal liver metastases (CLM) are the two most common malignant liver tumors. Hepatic resection (HR) is the only curative option, but only 15-20% of patients with liver metastases from CRC (CRLMs) are suitable for surgical standard treatment (1). For the HCC group, less than 30% of patients with HCC are eligible for surgery, mainly because of the multiplicity of the lesions that often occurs in a background of chronic liver disease, bad liver function, and deteriorating general condition (2,3).
Several alternative treatments to control and potentially cure have been developed for use in patients with malignant liver tumors, whether primary or metastatic. Interventional therapies, such as percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), microwave ablation (MWA) and Nano knife has been developed for treating malignant liver tumors.
RFA has gained wide acceptance by showing superior anticancer effects with low complications and mortality rate. Recently other emerging techniques such as MWA have attracted interest in clinical practice (4). However, these procedures will always entail some risks. Information regarding mortality and complications is absolutely essential for every intervention to permit an accurate assessment of the risks and benefits (5).
One of the greatest persistent problems in hepatic ablation has been the inability to establish quality standards in ablation complications, success, local recurrence after ablation, and nonablation hepatic recurrence. Reports from the literature are heterogeneous because of the study design, sample size, different technical approaches, and number of centers reporting complications and non-uniform terms as well as different parameters to calculate the rate of complications (6-9).
Major complications were defined as any symptom that developed after ablation and persisted for more than 1 week, or those that delayed hospital discharge, threatened the patient’s life, or led to substantial morbidity and disability (10). Major complication: included death, hemorrhage, RFA needle-track seeding, intra hepatic arterial pseudo aneurysm, RFA lesion abscess, perforation of gastrointestinal viscus, liver failure, biloma, biliary stricture, portal vein thrombosis, and hemothorax or pneumothorax requiring drainage, and minor complications including pain, fever, and asymptomatic pleural effusion.
Our goal was to bring the most updated literature regarding current used techniques (“what we really do”). The use of PEI has become less favorable in the face of new modalities such MWA and Nano knife, hence we decided to remove this technique from this review.
Ablation can be done either percutaneous or by surgery, in order to minimize bias related to surgery we decided to include only papers with percutaneous technique.
Materials and methods
Inclusion criteria
Randomized controlled trials (RCTs) and nonrandomized comparative studies assessing HCC or CRLM treated with RFA, MWA or Nano knife treatment were considered for review. Only patients aged over 18 were included. In order to exclude small studies, we only considered studies analyzing more than 50 patients for at least one technique.
Search strategy
A literature search was conducted on PubMed and EMBASE to identify clinical series of RFA, MWA and Nano knife procedures for liver tumours published between January 2000 and January 2014. Letters to the editors, supplements, review articles and case reports were excluded. The titles and abstracts of all potentially relevant trials were screened by one reviewer (LE). The full text articles of potentially relevant studies were obtained. Based on the full text article, another reviewer (HA) independently determined whether the study meets the inclusion/exclusion criteria.
Data collection
Information extracted from each study included: the number of patients, age and Child-Pugh score. The type of study were categorized as prospective, retrospective, observational or randomized trial and the type of intervention included RFA/MWA, the tumor according to type (HCC or metastasis). We extracted the data type for outcome measure using number of deaths, major complications and the description of the type of percutaneous ablative technique used.
Assessment of complications
In this study complications were reported in accordance with the guidelines recommended by the Working Group on Image-Guided Tumor Ablation (10).
The definition of major complication is an event that leads to substantial morbidity and disability, increasing the level of care, or results in hospital admission or substantially lengthened hospital stay (SIR classifications C-E) (Table 1). This includes any case in which a blood transfusion or interventional drainage procedure is required. All other complications are considered minor. It is important to stress that several complications, such as pneumothorax or tumor seeding, can be either a major or minor complications.

Full table
Results
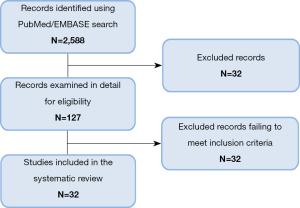
The search on Medline and EMBASE databases provided a total of 2,588 citations (Figure 1). After screening title and abstract, 2,461 were discarded. The full text of the remaining 127 citations was examined in more detail, where 95 studies did not meet the inclusion criteria as described. Finally 32 publications were included in the review.
Characteristics
Study design, participants and interventions
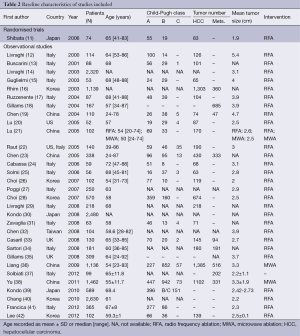
Of the 32 studies selected for the review, one was randomized trials and 31 were observational studies (Table 2). All the reports were published after 2000 (n=32). There were 29 studies using RFA only and 2 using MWA only. One observational study evaluated RFA versus MWA.
Full table
The included studies involved 15,744 participants. According to the type of technique, 13,044 and 2,700 patients were included for RFA and MWA respectively. The average age of patients ranged from 24 to 89 years. Mean tumor size treated ranged from 1.8 to 5.0 cm. In 16 studies, mortality and complications were primary outcomes.
Specific outcomes
Death and adverse events were assessed as secondary outcomes in 16 studies. Mean follow-up after treatment ranged from 10 to 137 months. For all percutaneous ablative techniques analyzed, mortality ranged from 0% to 0.88% and the pooled proportion was 0.16% (95% CI, 0.10-0.24%) by the random effects model. Individual analysis showed a pooled mortality of 0.15% for RFA, and 0.23% for MWA.
Major complication rates were 4.1% and 4.6% for RFA and MWA respectively.
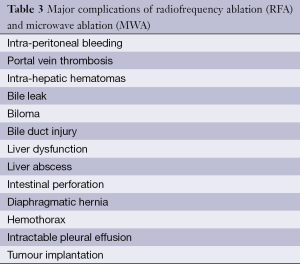
The most frequent major complication was hemorrhage intraperitoneal, subcapsular, pleural, biliary and retroperitoneal hemorrhage requiring blood transfusion (Table 3). Meanwhile the minor complication rates were 5.9% and 5.7% for RFA and MWA. There was no statistically significant difference in the mortality rates, major complications, and minor complications between the RFA and MWA groups (P>0.05).
Full table
Discussion
Ablation techniques have gained wide acceptance as a safe alternative to surgery in the management of early HCC and metastatic liver tumors (43,44).
The effectiveness of RFA in the treatment of malignant liver tumors has been proven by a number of clinical studies and medical practice reports (45-48). Recently, developments in MWA technology have demonstrated its unique advantage (49,50). Although we intended to include Nano knife in our review there are no publications up to now that met our inclusion criteria and a solid conclusion could not be excreted.
Post ablation complications such as liver failure, intraperitoneal bleeding, abscess, bile duct injury, tumor seeding are very serious, and can be life threatening (51,52), other complication can prolonged hospitalization and increase morbidity. Being well aware of the complications and the choice of treatment method will lead to a more practical application and enable this procedure to be safer and more effective.
The results without heterogeneity show a mortality of 0.15% and 0.23% for RFA and MWA, respectively. The prevalence of major complications in the reported studies ranged from 1.52% to 4.7%, calculated by using a random effects model in the presence of significant heterogeneity, were 4.1% for RFA and 4.6% for MWA.
MWA-associated mortality was reported to occur in 0.002% according to a systematic review of this technique (53). Major complication rates have been reported to be higher with MWA than with RFA in a randomized trial. Our results indicated that MWA is a safe technique in terms of mortality and major complication rate. However, the results should be interpreted with caution and more reports including large number of patients are needed to make a solid conclusion.
The difference between the reported complication rates can be explained by several factors: single/multicenter studies, prospective/retrospective studies.
Prospective studies may report more accurately the number of participants lost to follow-up, the timing of collecting complications and the adequate predefined definitions for harms.
It is well understood that the risk of complications can be reduced by proficiency in technique and refinement in pretreatment assessments.
There are several strategies for decreasing complications after ablation of hepatic tumors (51). The first key strategy is prevention by not to perform ablation in patients at high risk, meticulous pre evaluation of candidates should be performed, especially in regard to coagulopathy, underlying hepatic reserve, and tumor proximity to major structures such as the bile duct or intestine. In a patient with correctable coagulopathy, ablation should be postponed until all parameters are corrected.
Early detection cannot reduce the frequency of complications such as infection or bleeding, but it can potentially minimize their clinical magnitude. Thus, the operator and other medical personnel should be knowledgeable about the spectrum of various complications after ablation because complications can be detected even during the procedure in some cases. Close immediate follow-up with clinical and laboratory data is also essential for early detection of complications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vanagas T, Gulbinas A, Pundzius J, et al. Radiofrequency ablation of liver tumors (I): biological background. Medicina (Kaunas) 2010;46:13-7. [PubMed]
- Verslype C, Van Cutsem E, Dicato M, et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann Oncol 2009;20 Suppl 7:vii1-vii6. [PubMed]
- Zhu AX. Molecularly targeted therapy for advanced hepatocellular carcinoma in 2012: current status and future perspectives. Semin Oncol 2012;39:493-502. [PubMed]
- Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010;52:762-73. [PubMed]
- Lencioni R, Crocetti L, De Simone P, et al. Loco-regional interventional treatment of hepatocellular carcinoma: techniques, outcomes, and future prospects. Transpl Int 2010;23:698-703. [PubMed]
- Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg 2004;239:450-8. [PubMed]
- de Baère T, Risse O, Kuoch V, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 2003;181:695-700. [PubMed]
- Kasugai H, Osaki Y, Oka H, et al. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology 2007;72 Suppl 1:72-5. [PubMed]
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201-9. [PubMed]
- Goldberg SN, Charboneau JW, Dodd GD 3rd, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology 2003;228:335-45. [PubMed]
- Shibata T, Shibata T, Maetani Y, et al. Radiofrequency ablation for small hepatocellular carcinoma: prospective comparison of internally cooled electrode and expandable electrode. Radiology 2006;238:346-53. [PubMed]
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000;214:761-8. [PubMed]
- Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol 2001;11:914-21. [PubMed]
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441-51. [PubMed]
- Guglielmi A, Ruzzenente A, Battocchia A, et al. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology 2003;50:480-4. [PubMed]
- Rhim H, Yoon KH, Lee JM, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics 2003;23:123-34; discussion 134-6. [PubMed]
- Ruzzenente A, Manzoni GD, Molfetta M, et al. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol 2004;10:1137-40. [PubMed]
- Gillams AR, Lees WR. Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur Radiol 2004;14:2261-7. [PubMed]
- Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology 2004;232:260-71. [PubMed]
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005;41:1130-7. [PubMed]
- Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005;40:1054-60. [PubMed]
- Raut CP, Izzo F, Marra P, et al. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol 2005;12:616-28. [PubMed]
- Chen MH, Yang W, Yan K, et al. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol 2005;11:6395-401. [PubMed]
- Cabassa P, Donato F, Simeone F, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term experience with expandable needle electrodes. AJR Am J Roentgenol 2006;186:S316-21. [PubMed]
- Solmi L, Nigro G, Roda E. Therapeutic effectiveness of echo-guided percutaneous radiofrequency ablation therapy with a LeVeen needle electrode in hepatocellular carcinoma. World J Gastroenterol 2006;12:1098-104. [PubMed]
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol 2007;17:684-92. [PubMed]
- Poggi G, Riccardi A, Quaretti P, et al. Complications of percutaneous radiofrequency thermal ablation of primary and secondary lesions of the liver. Anticancer Res 2007;27:2911-6. [PubMed]
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol 2007;14:2319-29. [PubMed]
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82-9. [PubMed]
- Kondo Y, Yoshida H, Tateishi R, et al. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg 2008;95:996-1004. [PubMed]
- Zavaglia C, Corso R, Rampoldi A, et al. Is percutaneous radiofrequency thermal ablation of hepatocellular carcinoma a safe procedure? Eur J Gastroenterol Hepatol 2008;20:196-201. [PubMed]
- Chen TM, Huang PT, Lin LF, et al. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol 2008;23:e445-50. [PubMed]
- Casaril A, Abu Hilal M, Harb A, et al. The safety of radiofrequency thermal ablation in the treatment of liver malignancies. Eur J Surg Oncol 2008;34:668-72. [PubMed]
- Sartori S, Tombesi P, Macario F, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology 2008;248:670-9. [PubMed]
- Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206-13. [PubMed]
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology 2009;251:933-40. [PubMed]
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265:958-68. [PubMed]
- Yu J, Liang P, Yu XL, et al. Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol 2012;81:2495-9. [PubMed]
- Kondo Y, Shiina S, Tateishi R, et al. Intrahepatic bile duct dilatation after percutaneous radiofrequency ablation for hepatocellular carcinoma: impact on patient’s prognosis. Liver Int 2011;31:197-205. [PubMed]
- Chang IS, Rhim H, Kim SH, et al. Biloma formation after radiofrequency ablation of hepatocellular carcinoma: incidence, imaging features, and clinical significance. AJR Am J Roentgenol 2010;195:1131-6. [PubMed]
- Francica G, Saviano A, De Sio I, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis 2013;45:336-41. [PubMed]
- Lee HS, Park SY, Kim SK, et al. Thrombocytopenia represents a risk for deterioration of liver function after radiofrequency ablation in patients with hepatocellular carcinoma. Clin Mol Hepatol 2012;18:302-8. [PubMed]
- Khan MR, Poon RT, Ng KK, et al. Comparison of percutaneous and surgical approaches for radiofrequency ablation of small and medium hepatocellular carcinoma. Arch Surg 2007;142:1136-43; discussion 1143. [PubMed]
- Guglielmi A, Ruzzenente A, Valdegamberi A, et al. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg 2008;12:192-8. [PubMed]
- Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg 2009;249:20-25. [PubMed]
- Yan K, Chen MH, Yang W, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol 2008;67:336-347. [PubMed]
- Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol 2007;10:38-46. [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [PubMed]
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007;72 Suppl 1:124-31.. [PubMed]
- Jones C, Badger SA, Ellis G. The role of microwave ablation in the management of hepatic colorectal metastases. Surgeon 2011;9:33-7. [PubMed]
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441-51. [PubMed]
- Giorgio A, Tarantino L, de Stefano G, et al. Complications after percutaneous saline-enhanced radiofrequency ablation of liver tumors: 3-year experience with 336 patients at a single center. AJR Am J Roentgenol 2005;184:207-11. [PubMed]
- Ong SL, Gravante G, Metcalfe MS, et al. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol 2009;21:599-605. [PubMed]