Techniques for prevention of pancreatic leak after pancreatectomy
Introduction
Pancreatic resections, whether for benign or malignant disease processes, are some of the most technically challenging operations performed by surgeons. After pancreatic resection the potential for the development of serious complications exists. One of the most serious complications after pancreatic resection is the development of a post-operative pancreatic leak or fistula, whereby digestive pancreatic enzymes leak out of the pancreatic ductal system via an abnormal connection into the peri-pancreatic space or the peritoneal cavity, with resulting morbidity such as abdominal pain, ileus, fever, and the possibility of abscess, sepsis, and hemorrhage and consequently prolonged hospitalization. Importantly, patients with post-operative pancreatic fistula (POPF), leak, or abscess have been found to have a 90-day mortality of 5% in a single-institution report of pancreatectomy outcomes prospectively-collected over a five-year period (1). The magnitude of this complication is not insignificant; in a large worldwide literature search, the incidence of pancreatic fistula after pancreaticoduodenectomy was found to be 12.9% and 13% after distal pancreatectomy (2), and other reports detail fistula rates up to 31% for distal pancreatectomies (3).
Given the need to decrease the incidence of POPF as well as the resulting significant morbidity and mortality, various techniques have been attempted to prevent the formation of pancreatic leak and fistula. In this report we review techniques for the prevention of pancreatic leak after pancreatectomy.
Definition
A POPF is any abnormal connection between the pancreatic ductal system and the peri-pancreatic space, the peritoneal cavity or other body cavities, or externally to the skin. Leakage of enzyme-rich pancreatic fluid is typically diagnosed in the post-operative period via percutaneous drainage of a fluid collection that is found to be high in amylase content or via continued drainage of amylase-rich fluid through a drain placed at the time of surgery. In the past, varying criteria for what constitutes POPF have been published in the literature; however in an attempt to standardize the definition of POPF an international study group (ISGPF) of pancreatic surgeons convened in 2005 (4). POPF was thus defined as drain output of any volume occurring on or after post-operative day 3 with amylase content at least three times that of serum amylase levels.
In order to standardize the reporting of POPF outcomes, the authors also defined three grades of POPF: Grade A is a transient fistula that does not have any clinical impact, does not delay hospital discharge, and is managed by slow removal of peri-pancreatic drains. Grade B POPF requires a change in clinical management, such as making the patient NPO, administering TPN, or re-positioning drains, and leads to a delay in hospital discharge or to a readmission. Grade C POPF is the most severe and requires a major change in clinical management such as ICU-level care, percutaneous drainage of undrained fluid collections, or operative re-exploration for further drainage or attempted anastomotic repair. Grade C POPF causes a major increase in hospitalization time as well as increased rates of complications and the possibility of mortality (4).
Techniques to prevent pancreatic leak
Multiple trials using various operative techniques and pharmacologic agents have been conducted to evaluate for a decrease in or prevention of POPF. Herein we review the literature on techniques to decrease POPF.
Operative anastomotic construction techniques
Historical technique: ligation of the pancreatic duct
Historically the creation of a pancreatic-enteric anastomosis after pancreaticoduodenectomy was fraught with leak and complications, and thus some authors advocated simply ligating the pancreatic duct without re-creating continuity to the GI tract as a means of fistula prevention. Brunschwig reported on three cases of pancreatic duct ligation all without fistula creation in 1952 (5), and in a large report by Goldsmith and colleagues the POPF rate was equivalent between 45 patients treated with pancreatic duct ligation and 34 treated with anastomosis to the jejunum (6). Pancreatic endocrine dysfunction in the form of diabetes may develop after pancreatic duct ligation (7), and since approximately 1975 pancreatic duct ligation has been abandoned in favor of re-establishment of continuity of the pancreatic duct to the intestines (8,9).
Pancreaticojejunostomy (PJ) anastomotic techniques
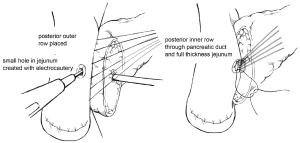
Multiple techniques in anastomosing the pancreatic duct to the gastrointestinal (GI) tract after pancreaticoduodenectomy have been described in the literature. Two of the predominant methods of creating a PJ are an end-to-side duct-to-mucosa anastomosis or the invagination technique. Briefly, in the end-to-side duct-to-mucosa anastomotic technique, the jejunal limb is brought into the retroperitoneum adjacent to the pancreas in a retrocolic fashion. A two-layer anastomosis is constructed with interrupted absorbable suture material, beginning with a posterior row of seromuscular sutures securing the jejunum to the pancreas (Figure 1). The pancreatic duct-to-mucosa anastomosis is performed to an enterotomy in the jejunum with a second circumferential layer of interrupted sutures, taking generous amounts of pancreas and the full-thickness of the jejunum, followed by completion of an anterior layer of seromuscular sutures again securing the anterior aspect of the opened jejunum to the capsule of the pancreas. In a report by Z’graggen and colleagues using this technique, POPF was seen in 2.1% of 331 patients who underwent pancreatic head resection (10).
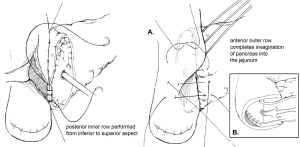
The goal of creating an invagination PJ is to invaginate or “dunk” all of the cut edge of the pancreatic parenchyma into the lumen of the jejunum (11). The performance of invagination PJ anastomosis begins with a posterior row of interrupted seromuscular sutures bringing the jejunum into apposition with the pancreatic capsule (Figure 2). The jejunum is opened, and an inner layer of running locking suture is then performed taking full-thickness jejunal bites and large bites of the pancreatic parenchyma and capsule, but not of the pancreatic duct, with the goal of invaginating all of the cut edge of the pancreatic tissue into the jejunum. An anterior layer of seromuscular sutures rolling the jejunum onto the pancreatic capsule is then performed to complete the anastomosis.
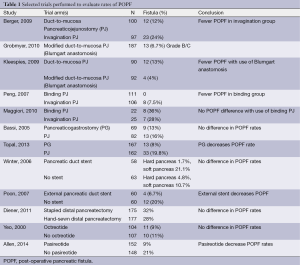
Berger and colleagues sought to compare rates of POPF at the PJ with the use of the invagination technique versus the duct-to-mucosa technique to test the hypothesis that use of the duct-to-mucosa technique would lead to a decreased POPF rate (12). To this end the authors performed a randomized prospective clinical trial at two institutions and randomized 197 patients undergoing pancreaticoduodenectomy to the invagination or the duct-to-mucosa technique; patients were stratified in both groups by whether the pancreatic parenchyma was hard or soft. POPF occurred in 17.8% of all patients, with significantly more POPF seen in the duct-to-mucosa group compared with the invagination group (24% vs. 12%, P<0.05) and with more POPF in soft glands (27%) than in hard glands (8%). The authors concluded that the pancreatic texture was the greatest determinant in POPF and that further studies are needed to determine the optimal anastomotic technique.
Modified duct-to-mucosa PJ
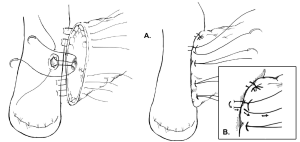
One variation of the duct-to-mucosa technique that bears noting is the transpancreatic U-suture technique with a duct-to-mucosa anastomosis described by Grobmyer, Blumgart, and colleagues at Memorial Sloan Kettering Cancer Center and originally created by Dr. Leslie Blumgart (13). In this technique an outer layer of polyglactin sutures are first inserted full-thickness anterior-to-posterior through the pancreas with subsequent seromuscular horizontal mattress stitches on the jejunum, followed again by a full-thickness posterior-to-anterior bite coming up through the pancreas (Figure 3). Care is taken not to pass the needle through the pancreatic duct. The u-stitches are not tied yet, and a duct-to-mucosa anastomosis is then created with fine polydioxanone interrupted suture. The seromuscular sutures are then tied bringing the jejunum into close apposition anteriorly on the pancreas; however the suture is not yet cut. Lastly, the sutures with the needles still on are used to create an anterior seromuscular bite on the jejunum with the needle being brought through the pancreas under the previous knots. The sutures are then tied again, thus imbricating the jejunum over the entire pancreas. In an audit of 187 patients with PJ anastomoses constructed by this technique, the authors report an overall POPF rate of 20.3%; however most of these were ISPGF Grade A, with only 6.9% of patients with Grade B or C POPF. Soft pancreatic texture was significantly associated with leak, and patients with POPF had significantly smaller diameter pancreatic ducts compared with patients without POPF (3 vs. 4 mm, P=0.008). Kleespies and colleagues published their outcomes data using what they call the “Blumgart anastomosis” after their department began to use this technique for PJ and abandoned the traditional duct-to-mucosa technique (14). They found significantly decreased leak rate with the Blumgart anastomosis (13% vs. 4%, P=0.032), as well as significantly decreased rates of postoperative hemorrhage, complications, and length of ICU stay. Proponents of this technique argue that the transpancreatic sutures minimize radial forces on the anastomosis, and that it is relatively quick to construct and easy to teach to trainees.
Binding technique for PJ creation
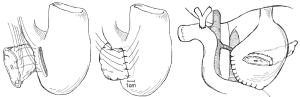
Another technique for creating the PJ anastomosis is the so-called “binding” PJ reported by Peng and colleagues (15), in which the distal 3 cm of the jejunal loop to be used for anastomosis are everted and the mucosa ablated either by electrocoagulation or by topical treatment with 10% carbolic acid followed by immediate rinsing in 75% ethanol and normal saline (Figure 4). The proximal 3 cm of the pancreatic stump is then anastomosed to just the mucosa of the jejunum. The treated 3 cm of jejunum are then rolled out and intussuscepted back over the pancreas, sutured into place, and lastly a catgut tie is looped around the entire circumference of the anastomosis 1 cm from the cut edge of the pancreas. The authors reported a 0% POPF rate after the completion of 150 cases using this anastomosis, with an overall morbidity of 31.3% and a mean hospital stay of 19.8±5 days (16). A subsequent prospective trial conducted by Peng and colleagues randomized 217 patients undergoing pancreaticoduodenectomy to traditional PJ anastomosis or binding PJ anastomosis (17). Leak was seen in 8 of 111 (7.2%) conventional PJ patients compared with 0 of 106 binding PJ patients (P=0.014), and complications were reported in 36.9% of conventional PJ patients compared with 24.5% of binding PJ patients (P=0.048), including 6.3% perioperative mortality in the conventional group and 2.8% mortality in the binding group (P=NS). Subsequent trials of binding PJ conducted in Europe have not replicated the impressive rate of POPF. A case-control study 22 binding PJ and 25 conventional PJ patients found no difference in the rate of POPF, with longer delay in POPF healing as well as increased postpancreatectomy hemorrhage in the binding group (18). Similarly, a recent prospective two-institution trial of 69 binding PJ patients compared to 52 conventional PJ historical control patients demonstrated significantly shorter hospital stay in the conventional PJ patients. Soft pancreatic texture was significantly associated with POPF; however no significant difference in the rate of POPF between binding and conventional PJ anastomoses was seen (19). Binding PJ remains one of many options for creation of the pancreatic-enteric anastomosis.
Pancreaticogastrostomy (PG) creation
The creation of pancreatic duct anastomosis to the stomach PG instead of to the jejunum has been studied as well, with the rationale that a PG anastomosis is easier to perform and that the stomach has a more robust blood supply compared with the jejunum. Additional rationale for PG instead of PJ in the case of pancreatic head resections that extend to the left past the midline is that the increase in distance may put the resulting jejunal limb and jejunal anastomosis under tension, with increased risk for subsequent leak; however after such a resection the stomach will be immediately adjacent to the remnant pancreas with the opportunity to create a tension-free PG anastomosis (Figure 5). In evaluating PG, an earlier report by Delcore and colleagues demonstrated no leaks of the PG anastomosis in 45 cases (20), and a 0% leak rate over 38 cases was also reported by Mason et al. (21). PG was later compared to PJ anastomosis in a prospective randomized trial conducted by Bassi and co-workers, in which 151 patients with soft pancreatic glands were randomized to PG or end-to-side PJ anastomoses (22). Pancreatic fistula occurred in 13% of PG patients and 16% of PJ (P=NS); however post-operative fluid collections, delayed gastric emptying, and biliary fistulae were significantly less in the PG group. A similar trial was conducted by Duffas et al. who randomized 81 patients to PG and 68 patients to PJ after pancreaticoduodenectomy and found POPF in 16% of the PG group and 20% of the PJ group (23). The authors concluded that the type of anastomosis does not influence the development of POPF, and a meta-analysis of PG versus PJ trials noted that there was no superiority of either technique and surgeons should continue to use the technique with which they are most familiar (24). Interestingly, a recent prospective randomized multi-center trial by Topal and colleagues from Belgium randomizing 329 patients to PJ or PG after pancreaticoduodenectomy, in which patients were stratified by pancreatic duct diameter (≤3 or >3 mm), reported significantly more POPF in the PJ group than the PG group (19.8% vs. 8%, OR 2.86, 95% CI: 1.38-6.17, P=0.002) (25). The authors concluded that PG should be the preferred anastomosis after pancreaticoduodenectomy, although further data from a multi-center international trial will be needed to confirm this.
Pancreatic duct anastomotic stenting
Pancreatic duct stenting at the time of anastomosis creation has been proposed as a technique to decrease pancreatic leak and fistula, with the rationale that stenting prevents the accumulation of pancreatic secretions in the pancreatic stump and the pancreatic anastomosis is excluded from direct contact with the pancreatic juice (26). This was examined in a randomized trial by Winter and colleagues who randomized 238 patients undergoing pancreaticoduodenectomy to internal pancreatic duct stent or no-stent with the endpoint of POPF development (27). Patients were stratified by the texture of the pancreatic remnant (soft vs. normal/hard), with 6 cm pediatric feeding tubes were used as stents. In the hard pancreas group 1.7% stent patients and 4.8% non-stent patients developed POPF (P=0.4), and in the soft pancreas group 21.1% stent patients and 10.7% non-stent patients developed POPF (P=0.1) with the conclusion that internal pancreatic duct stenting does not alter the rate of POPF.
Pancreatic duct drainage with external rather than stents has also been studied. In a study from Hong Kong in 2007, Poon et al. prospectively randomized 120 patients undergoing pancreaticoduodenectomy with PJ duct-to-mucosa anastomosis to an external stent or not (28). Patients in the stented group had a significantly lower pancreatic fistula rate compared with the no stent group (6.7% vs. 20%, P=0.032), and on multivariable analysis absence of stenting was a significant risk factor for POPF. The authors hypothesized that use of external drains more completely diverts pancreatic secretions away from the PJ anastomosis with decreased risk for leak formation.
A recent Cochrane Review also examined the efficacy of pancreatic stents in preventing POPF after pancreaticoduodenectomy in a review of randomly controlled trials extracted the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Excerpta Medica database (EMBASE), Web of Science, and other major trials databases (29). A total of 655 patients were included in the systematic review, and the authors found that the use of external, but not internal stents was associated with a significant decrease in the incidence of POPF (RR 0.33, 95% CI: 0.11-0.98, P=0.002). These results are echoed by another systematic review of the literature and meta-analysis performed by Xiong and colleagues, who examined the literature from January 1973 to September 2011 and included 1,726 patients from five randomized clinical trials and 11 non-randomized clinical observation studies in their analysis (30). The authors found that placement of internal or external stents in the pancreatic duct after pancreaticoduodenectomy did not reduce the incidence of POPF; however on subgroup analysis placement of external stents significantly reduced the incidence of POPF compared with no stent (OR 0.42, 95% CI: 0.24-0.76, P=0.004 for randomized clinical trials and OR 0.43, 95% CI: 0.27-0.68, P<0.001 for observational studies). These recent data suggest that if one intends to stent the pancreatic anastomosis, an external stent should be considered; however more data are needed to suggest routine use of pancreatic stents, and many centers have moved away from the use of pancreatic duct stents completely.
Pancreatic stump closure after distal pancreatectomy
Pancreatic leak after distal pancreatectomy occurs in approximately 30% of patients (31,32), which is a rate higher than is seen in pancreaticoduodenectomy. Many studies have been conducted to determine the optimal method for closing the pancreatic stump in order to prevent POPF. The two main techniques for closure of the pancreatic stump after distal pancreatectomy are suture closure of the pancreatic duct or stapled closure of the parenchyma. A previous retrospective report by Bilimoria et al. in which the authors reviewed their institutional data of 126 patients who underwent distal pancreatectomy over a nine year period found that POPF rates in patients who underwent suture closure of the pancreatic duct was significantly lower than patients who did not undergo suture closure (9.6% vs. 34%, P<0.001) (33). On multivariable analysis, failure to ligate the duct was significantly associated with pancreatic leak (OR 5, 95% CI: 2-10, P=0.001). The other most prominent technique for pancreatic transection is to use a surgical stapler. A meta-analysis conducted by Knaebel and co-workers in 2005 examined ten articles in the world literature (two randomized trials and eight observational studies) that reported techniques to decrease POPF after distal pancreatectomy (34). Six of the ten studies compared hand-sutured versus stapled pancreatic closure, and in this analysis the authors found a trend towards decreased POPF with the use of staplers; however the results were not statistically significant (OR 0.66, 95% CI: 0.35-1.26, P=0.21). Given this trend towards decreased POPF with stapled closure, Diener and colleagues designed the multicenter prospective DISPACT trial in which patients undergoing distal pancreatectomy were randomized to stapler or hand-sewn closure with the primary outcomes of POPF and mortality at one week; the authors hypothesized that standardized closure with a stapler would lead to decreased POPF (35). Of 450 patients randomized, 352 were included in the final analysis (175 hand-sewn, 177 stapler). The rate of POPF in the stapler group was 32% compared with 28% in the hand-sewn group, without any significant difference between the two groups (OR 0.84, 95% CI: 0.53-1.33, P=0.56). There was one death in the hand-sewn group and none in the stapler group. The authors concluded that stapled closure was not superior to hand-sewn closure for preventing POPF, and indeed the data demonstrate that these methods of closure have equivalent POPF rates.
Given this equivalency, other methods to decrease POPF have been investigated. A prospective randomized trial of prophylactic pancreatic duct stenting to decrease POPF was conducted by Frozanpor et al. with the hypothesis that more efficient diversion of pancreatic secretions into the duodenum away from the pancreatic transection line would lead to decreased POPF (36). A total of 58 patients were analyzed (29 distal pancreatectomy only, 29 distal pancreatectomy with stent); the rate of ISGPF Grade B/C POPF was 42.3% in the stent group and 22.2% in the no-stent group without a significant difference between the two (OR 2.57, 95% CI: 0.78-8.48, P=0.122). Decreasing resistance across the sphincter of Oddi with stenting does not appear to have a role in decreasing POPF rates.
Various methods of reinforcing the staple line after distal pancreatectomy have been attempted as a means of decreasing leak. In a small non-randomized single-institution trial, Jimenez and colleagues reported rates of POPF with stapled pancreatic stump closure reinforced with bioabsorbable buttress sleeves mounted on the stapler and compared a group of 13 patients treated in this manner with 18 historical controls (37). Rates of POPF were 0% in the buttress group versus 39% in the control group (P=0.025). A similar single-institution report from Thaker and others of 40 patients undergoing distal pancreatectomy and bioabsorbable mesh buttress staple line reinforcement with comparison to 40 historical controls of only stapled closure found significantly decreased rate of POPF with mesh reinforcement (3.5%) compared with staple closure only (22%, P=0.04) (38). In a subsequent single-institution randomized prospective trial of stapled pancreatic closure with or without bioabsorbable mesh staple line reinforcement, Hamilton et al. found significantly fewer ISGPF Grade B/C leaks in 1/53 (1.9%) mesh reinforcement patients compared with 11/45 (20%) no-mesh patients (P=0.007) (39).
Currently it appears that reinforcement of the pancreatic staple line with a bioabsorbable mesh is a feasible method of decreasing POPF; however the previous single-institution results still require confirmation in the form of multi-institution prospective randomized trials, preferably with international collaboration. Just as the rigorous methodology of the DISPACT trial appears to have provided a definitive answer to the question of stapled or hand-sewn closure, so is there a need for this methodology regarding the question of bioabsorbable mesh reinforcement.
Use of fibrin glue and other topical sealant agents
The use of fibrin glue and other topical hemostatic agents applied to the pancreato-enteric anastomosis have been proposed as adjuncts to help seal the anastomosis and prevent POPF; however results have been disappointing. In a report from 1991, Kram and colleagues used fibrin glue made from concentrated fibrinogen and clotting factors which was applied topically to pancreatic wounds, staple/suture lines, and pancreatic anastomoses in both trauma and non-trauma operations; the authors reported no pancreatic fistulae, abscesses, or pseudocysts in their series of 15 patients (40). In an early prospectively randomized trial reported in 1994 by D’Andrea, 97 patients undergoing pancreatectomy for both benign and malignant conditions were enrolled and randomized to intraoperative fibrin sealing of the pancreas or to no sealing (41). Pancreatic fistulae developed in 13.9% of the fibrin glue patients and in 11.1% of the non-fibrin glue patients, with no significant difference seen between in two groups.
In a larger prospective randomized trial of fibrin glue conducted by Lillemoe et al., the authors randomized 125 patients, who were felt to be at high risk for pancreatic leak after pancreaticoduodenectomy by their operating surgeon, to either topical application of fibrin glue to the PJ anastomosis (59 patients) versus no glue (66 patients) (42). The rate of POPF was 26% in the glue arm versus 30% in the control group (P=NS), and there was no difference in length of hospital stay between the groups as well. The authors concluded that the use of fibrin glue did not decrease the rate of POPF or of other complications following pancreaticoduodenectomy. A recent large meta-analysis evaluating the effectiveness of fibrin sealants in pancreatic surgery systematically evaluated seven studies including 897 patients and found that fibrin sealants had a non-significant impact on the development of POPF (43). The authors concluded that fibrin sealants cannot be recommended routinely in the setting of pancreatic resection.
Internal occlusion of the pancreatic duct with absorbable fibrin glue after creation of a pancreatic duct anastomosis has been proposed as a way to allow the anastomosis to heal without being exposed to the enzyme-rich pancreatic fluid, although early prospective non-randomized trials did not demonstrate a decrease in POPF (44). To address this, Suc and colleagues conducted a multi-institution, single-blind, prospective randomized trial in France of pancreatic resection with or without fibrin glue occlusion of the pancreatic duct occlusion (45). The authors report an overall POPF rate of 16% in their trial; however no difference in POPF rate was seen when comparing the fibrin glue to the control group. Fibrin glue occlusion of the pancreatic duct appears to have no impact on the development of POPF.
Use of somatostatin analogues
The inhibitory peptide hormone somatostatin acts to decrease the output of secretions from the pancreas, GI tract, and biliary tract, although the half-life is short at approximately two minutes (46). Synthetic analogues of somatostatin with longer half-lives, such as octreotide (47), have been developed and have been used in pancreatic surgery in an attempt to decrease POPF, with the hypothesis that decreased pancreatic juice secretion will allow for improved healing of pancreatic ductal anastomoses and consequently decreased leak rates. The use of octreotide has been studied in multiple randomized prospective trials in the United States and Europe; however the results have been mixed. Yeo and colleagues conducted a prospective trial in which patients undergoing pancreaticoduodenectomy were randomized to saline control or octreotide 250 µg subcutaneously every eight hours beginning 1-2 hours before surgery and continuing for seven days (48). Ultimately 211 patients made up the entire study cohort; POPF was seen in 9% of control group and 11% of octreotide group. The authors concluded that octreotide does not reduce incidence of POPF and that omission of this treatment may lead to a cost savings for hospitals. Sarr and co-investigators in the Pancreatic Study Group conducted a prospective, randomized, placebo-controlled trial of the long-acting somatostatin analogue vapreotide, hypothesizing that vapreotide would decrease pancreas-related complications; 135 patients received vapreotide and 140 received placebo (49). No significant differences were seen in pancreas-related complications between the two groups (placebo 26.4% vs. vapreotide 30.4%, P=NS), and the authors concluded that vapreotide offers no therapeutic benefit in terms of post-operative complications. Suc et al. conducted a French multi-center prospective randomized trial in 230 patients undergoing pancreatectomy, with 122 patients randomized to octreotide and 108 randomized to the control arm; the primary endpoint was all intra-abdominal complications (50). Intra-abdominal complications were seen in 22% of octreotide patients versus 32% of placebo patients; however this result was not statistically significant and the authors concluded that octreotide cannot be routinely used to decrease intra-abdominal complications in pancreatectomy patients.
Recently, Allen and colleagues reported their results of a single-center, prospective, double-blind, placebo controlled trial using the long-acting somatostatin analogue pasireotide, which has a longer half-life than octreotide as well as a broader receptor binding profile (51). Patients undergoing pancreaticoduodenectomy or distal pancreatectomy were randomized to pasireotide 900 µg subcutaneously given twice daily beginning the morning of operation for seven days (152 patients) or to placebo (148 patients). The primary endpoint was incidence of grade 3 pancreatic leak, fistula, or abscess; grade 3 indicating that a radiologic, endoscopic, or surgical intervention was required, and the secondary endpoint was Grade B or C POPF. In total 15% of patients met the primary endpoint; however the primary endpoint was significantly less in the pasireotide group compared with placebo (9% vs. 21%, RR 0.44, 95% CI: 0.24-0.78, P=0.006). In the pasireotide group 7.9% of patients had Grade B POPF, and zero had Grade C, compared with 16.9% Grade B/C in the placebo group, P=0.02; rates of adverse events were similar between the two groups. Pasireotide significantly reduced risk of post-operative fistula/leak/abscess, and may have a role in the prevention of POPF in the future.
Conclusions
Post-operative pancreatic leak and fistula are a major source of morbidity and mortality after pancreatic resection. Many trials have been undertaken to identify techniques to reduce POPF (Table 1); however no one technique has been shown to definitively be the solution to the problem, and indeed one of the major determinants of POPF is a factor over which the surgeon has very little control, i.e., the consistency of the pancreatic parenchyma itself. Surgeons should continue to use the pancreatic duct anastomotic technique with which they are most familiar and comfortable, and currently there is no evidence for routine use of stents or topical sealing products. For closure of the pancreatic stump after distal pancreatectomy, a stapled closure in combination with bioabsorbable mesh buttress may represent a reliable technique to decrease POPF; however high-quality data from multi-institutional prospective trials are currently lacking. In the future, novel somatostatin analogues may play a role in decreasing POPF, but without question meticulous surgical technique and attention to detail will remain the cornerstones of decreasing pancreatic leak and patient morbidity/mortality.
Full table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vin Y, Sima CS, Getrajdman GI, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg 2008;207:490-8. [PubMed]
- Alexakis N, Sutton R, Neoptolemos JP. Surgical treatment of pancreatic fistula. Dig Surg 2004;21:262-74. [PubMed]
- Goh BK, Tan YM, Chung YF, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg 2008;143:956-65. [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [PubMed]
- Brunschwig A. Pancreatoduodenectomy: a curative operation for malignant neoplasms in the pancreatoduodenal region; report of three over-five-year survivors. Ann Surg 1952;136:610-24. [PubMed]
- Goldsmith HS, Ghosh BC, Huvos AG. Ligation versus implantation of the pancreatic duct after pancreaticoduodenectomy. Surg Gynecol Obstet 1971;132:87-92. [PubMed]
- Tran K, Van Eijck C, Di Carlo V, et al. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg 2002;236:422-8; discussion 428. [PubMed]
- Bartoli FG, Arnone GB, Ravera G, et al. Pancreatic fistula and relative mortality in malignant disease after pancreaticoduodenectomy. Review and statistical meta-analysis regarding 15 years of literature. Anticancer Res 1991;11:1831-48. [PubMed]
- Fromm D, Schwarz K. Ligation of the pancreatic duct during difficult operative circumstances. J Am Coll Surg 2003;197:943-8. [PubMed]
- Z’graggen K, Uhl W, Friess H, et al. How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg 2002;9:733-7. [PubMed]
- Kennedy EP, Yeo CJ. Dunking pancreaticojejunostomy versus duct-to-mucosa anastomosis. J Hepatobiliary Pancreat Sci 2011. [Epub ahead of print].
- Berger AC, Howard TJ, Kennedy EP, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg 2009;208:738-47; discussion 747-9. [PubMed]
- Grobmyer SR, Kooby D, Blumgart LH, et al. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg 2010;210:54-9. [PubMed]
- Kleespies A, Rentsch M, Seeliger H, et al. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg 2009;96:741-50. [PubMed]
- Peng S, Mou Y, Cai X, et al. Binding pancreaticojejunostomy is a new technique to minimize leakage. Am J Surg 2002;183:283-5. [PubMed]
- Peng SY, Mou YP, Liu YB, et al. Binding pancreaticojejunostomy: 150 consecutive cases without leakage. J Gastrointest Surg 2003;7:898-900. [PubMed]
- Peng SY, Wang JW, Lau WY, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;245:692-8. [PubMed]
- Maggiori L, Sauvanet A, Nagarajan G, et al. Binding versus conventional pancreaticojejunostomy after pancreaticoduodenectomy: a case-matched study. J Gastrointest Surg 2010;14:1395-400. [PubMed]
- Casadei R, Ricci C, Silvestri S, et al. Peng’s binding pancreaticojejunostomy after pancreaticoduodenectomy. An Italian, prospective, dual-institution study. Pancreatology 2013;13:305-9. [PubMed]
- Delcore R, Thomas JH, Pierce GE, et al. Pancreatogastrostomy: a safe drainage procedure after pancreatoduodenectomy. Surgery 1990;108:641-5; discussion 645-7. [PubMed]
- Mason GR, Freeark RJ. Current experience with pancreatogastrostomy. Am J Surg 1995;169:217-9. [PubMed]
- Bassi C, Falconi M, Molinari E, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg 2005;242:767-71, discussion 771-3. [PubMed]
- Duffas JP, Suc B, Msika S, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg 2005;189:720-9. [PubMed]
- Dixon E, Fingerhut A, Bassi C, et al. Meta-analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy: authors' comment (Br J Surg 2006; 93: 929-936). Br J Surg 2006;93:1435. [PubMed]
- Topal B, Fieuws S, Aerts R, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol 2013;14:655-62. [PubMed]
- Kleespies A, Albertsmeier M, Obeidat F, et al. The challenge of pancreatic anastomosis. Langenbecks Arch Surg 2008;393:459-71. [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg 2006;10:1280-90; discussion 1290. [PubMed]
- Poon RT, Fan ST, Lo CM, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;246:425-33; discussion 433-5. [PubMed]
- Dong Z, Xu J, Wang Z, et al. Stents for the prevention of pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev 2013;6:CD008914. [PubMed]
- Xiong JJ, Altaf K, Mukherjee R, et al. Systematic review and meta-analysis of outcomes after intraoperative pancreatic duct stent placement during pancreaticoduodenectomy. Br J Surg 2012;99:1050-61. [PubMed]
- Ferrone CR, Warshaw AL, Rattner DW, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg 2008;12:1691-7; discussion 1697-8.
- Nathan H, Cameron JL, Goodwin CR, et al. Risk factors for pancreatic leak after distal pancreatectomy. Ann Surg 2009;250:277-81. [PubMed]
- Bilimoria MM, Cormier JN, Mun Y, et al. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg 2003;90:190-6. [PubMed]
- Knaebel HP, Diener MK, Wente MN, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg 2005;92:539-46. [PubMed]
- Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377:1514-22. [PubMed]
- Frozanpor F, Lundell L, Segersvärd R, et al. The effect of prophylactic transpapillary pancreatic stent insertion on clinically significant leak rate following distal pancreatectomy: results of a prospective controlled clinical trial. Ann Surg 2012;255:1032-6. [PubMed]
- Jimenez RE, Mavanur A, Macaulay WP. Staple line reinforcement reduces postoperative pancreatic stump leak after distal pancreatectomy. J Gastrointest Surg 2007;11:345-9. [PubMed]
- Thaker RI, Matthews BD, Linehan DC, et al. Absorbable mesh reinforcement of a stapled pancreatic transection line reduces the leak rate with distal pancreatectomy. J Gastrointest Surg 2007;11:59-65. [PubMed]
- Hamilton NA, Porembka MR, Johnston FM, et al. Mesh reinforcement of pancreatic transection decreases incidence of pancreatic occlusion failure for left pancreatectomy: a single-blinded, randomized controlled trial. Ann Surg 2012;255:1037-42. [PubMed]
- Kram HB, Clark SR, Ocampo HP, et al. Fibrin glue sealing of pancreatic injuries, resections, and anastomoses. Am J Surg 1991;161:479-81; discussion 482. [PubMed]
- D’Andrea AA, Costantino V, Sperti C, et al. Human fibrin sealant in pancreatic surgery: it is useful in preventing fistulas? A prospective randomized study. Ital J Gastroenterol 1994;26:283-6. [PubMed]
- Lillemoe KD, Cameron JL, Kim MP, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg 2004;8:766-72; discussion 772-4. [PubMed]
- Orci LA, Oldani G, Berney T, et al. Systematic review and meta-analysis of fibrin sealants for patients undergoing pancreatic resection. HPB (Oxford) 2014;16:3-11. [PubMed]
- Lorenz D, Wolff H, Waclawiczek H. Pancreatic duct occlusion in resection treatment of chronic pancreatitis and cancer of the head of the pancreas. A 3-year follow-up study. Chirurg 1988;59:90-5. [PubMed]
- Suc B, Msika S, Fingerhut A, et al. Temporary fibrin glue occlusion of the main pancreatic duct in the prevention of intra-abdominal complications after pancreatic resection: prospective randomized trial. Ann Surg 2003;237:57-65. [PubMed]
- Raptis S, Schlegel W, Lehmann E, et al. Effects of somatostatin on the exocrine pancreas and the release of duodenal hormones. Metabolism 1978;27:1321-8. [PubMed]
- Meier R, Dierdorf R, Gyr K. Somatostatin analog (octreotide) in clinical use: current and potential indications. Schweiz Med Wochenschr 1992;122:957-68. [PubMed]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000;232:419-29. [PubMed]
- Sarr MG, Pancreatic Surgery Group. The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: a prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg 2003;196:556-64; discussion 564-5; author reply 565.[PubMed]
- Suc B, Msika S, Piccinini M, et al. Octreotide in the prevention of intra-abdominal complications following elective pancreatic resection: a prospective, multicenter randomized controlled trial. Arch Surg 2004;139:288-94; discussion 295. [PubMed]
- Allen PJ, Gönen M, Brennan MF, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med 2014;370:2014-22. [PubMed]