Fascioliasis simulating an intrahepatic cholangiocarcinoma—Case report with imaging and pathology correlation
Introduction
Human fascioliasis, caused by a trematode that inhabits the bile ducts, is considered an emerging disease due to the increasing number of notifications of people infected by the species of the genus Fasciola. Which has a cosmopolitan distribution, and is present particularly in areas of sheep or cattle breeding associated with the presence of the intermediary host, a snail of the genus Lymnaea. In Latin America, the prevalence of fascioliasis is among the highest in the world, being endemic in humans and with numbers that vary widely, from 66.7% on Bolivia’s high plain to the lowest, close to 0.7% in the central zone of Chile. It is widely distributed in Chile, except in the XIIth Region due to its low temperatures (1). The aim of this work is to report a clinical case in which a patient infected by a trematode of the genus Fasciola presented with a tumor in the right hepatic lobe compatible with intrahepatic cholangiocarcinoma and to review the clinical presentation, imaging study, pathology result and treatment.
Clinical case
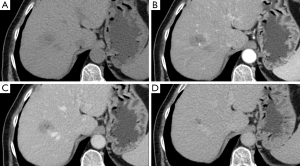
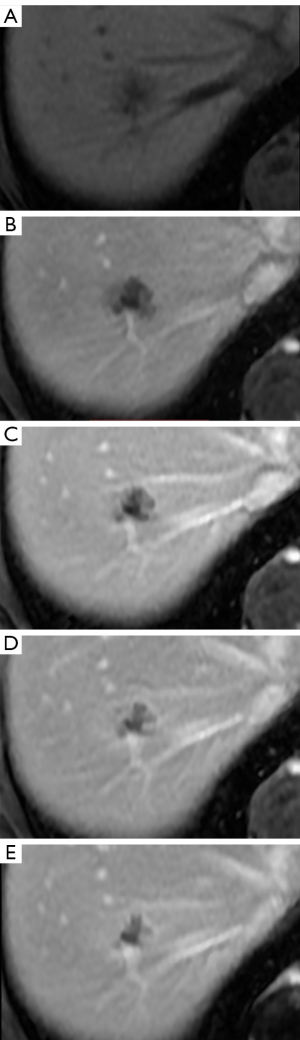
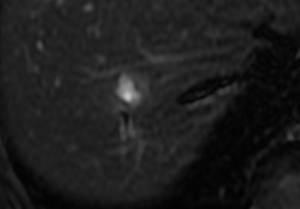
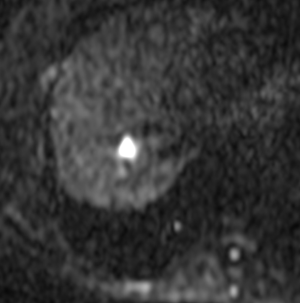
A female patient, 57 years of age, with a long history of gastroesophageal reflux treated with esomeprazole. She consulted gastroenterology for intense, intermittent night-time epigastric pain. Her initial clinical examination was unremarkable. The laboratory tests were as follows: hematocrit: 39.5%; leukocyte count 6,900 /uL; eosinophils: 5%; and erythrocyte sedimentation rate: 11 mm/h. The lipid profile, urine test and thyroid-stimulating hormone were normal. The ultrasound from 2 years prior to the consultation was normal. The upper gastrointestinal endoscopy showed mild erosive esophagitis, incompetence of the gastric cardia and antral erosive gastropathy. The rapid urease test for Helicobacter pylori was negative, and the esophageal biopsy revealed mild chronic esophagitis with no eosinophilic infiltration. A colonoscopy showed an 8 mm rectal polyp, inflammatory process of the sigmoids, sigmoid diverticulosis. The pathology of the polyp revealed an adenoma with high-grade dysplasia. The abdominal computed tomography (CT) scan showed a hypodense irregular focal lesion of 27 mm in the segment 8 of the right hepatic lobe, lobulated and hypovascular. The lesion had a weak peripheral enhancement progressing towards the center after administration of the intravenous iodized contrast. There was an eccentric irregularly shaped region with cystic characteristics in the most peripheral area of the lesion (Figure 1). A magnetic resonance imaging (MRI) with liver protocol confirmed the presence of the lesion seen in the CT. A T1-weighted hypointense lesion, presenting progressive enhancement with the gadolinium-based contrast medium was found (Figure 2). The area with no enhancement was interpreted as a focal dilatation of the intrahepatic bile duct (Figures 3 and 4). In addition, the lesion presented signal hyperintensity in the diffusion-weighed image (Figure 5). Neither capsular nor subcapsular alterations were identified in these studies. In light of these findings, the diagnostic possibility of intrahepatic cholangiocarcinoma was suggested. The tumor markers alpha-fetoprotein (2.31 ng/mL); Ca125 (4.84 U/mL); Ca19-9 (6.66 U/mL); carcinoembryonic antigen (7.21 ng/mL) were within normal limits.
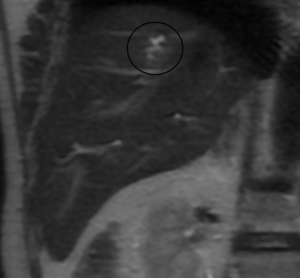
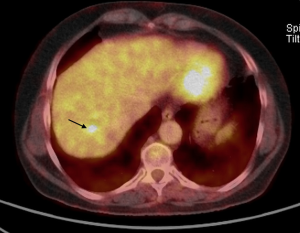
A study using positron emission tomography in combination with CT (PET-CT) with 18F-fluoro-2-deoxy-D-glucose (18 FDG) showed a single hypermetabolic center in relation to the hepatic mass (Figure 6).
The tumor board of the Clinica Alemana Temuco made the decision to perform a right hepatectomy in view of the suspicion of intrahepatic cholangiocarcinoma.
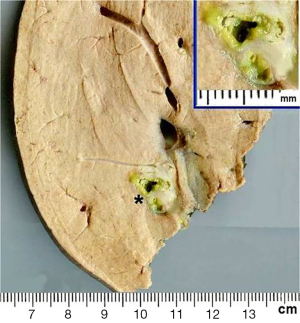
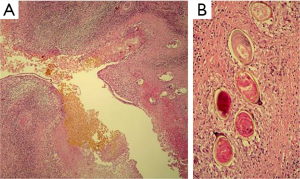
Seven weeks after the initial consultation, a limited right hepatectomy was carried out without complications. The gross examination of the surgical specimen showed a 1.7 cm × 1.3 cm ovoid lesion, with a whitish-greenish color, compatible with bile duct dilatation, 1.2 cm from surgical edge (Figure 7). The microscopy revealed normal hepatic tissue and portal triads with minimal lymphocyte infiltration. There was an inflammatory process outside and inside of the intrahepatic bile ducts (Figure 8A), an area with granulomas that contained bile and undrained necrotic material, lymphocytes, eosinophils, epithelioid cells and foreign body-type multinucleated giant cells in relation to ovoid structures of approximately 0.15 mm (Figure 8B). These structures were composed of hyaline capsules, some of which were collapsed, with amorphous, eosinophilic content suggestive of eggs of the genus Fasciola. Neither young nor adult parasitic forms were identified. The biliary epithelium was detached in some parts and in others it had regenerative atypias. No neoplasia was found. The histopathological study concluded: cholangitis and granulomatous pericholangitis due to parasitic eggs. The patient was discharged on the sixth postoperative day in good general condition, with no postoperative morbidity.
A follow-up MRI on the 10th postoperative day showed no collections or lesions.
The patient was referred to infectious diseases for anti-parasite treatment. She received a single oral dose of 10 mg of triclabendazole per kilogram of body weight (10 mg/kg).
Discussion
Fascioliasis is a zoonosis of cosmopolitan distribution produced by F. gigantica and F. hepatica. The latter is the most widely distributed globally. The leaf-shaped adults (3.5 cm long by 1 cm wide) inhabit bile ducts and the gallbladder. They produce unembryonated eggs in the bile ducts, which are eliminated in the stool. In an aquatic environment, the eggs release miracidia that invade the snail (intermediary host). After going through 3 larval states they free themselves from the snail and immature states encyst in the surface of vegetables, particularly watercress, which infects human beings and other herbivores upon consumption (2).
In the acute phase the symptoms are produced by the migration of the parasite from the intestine to the liver, and can include nausea, vomiting, abdominal pain, fever, rash and dyspnea. During the chronic phase (adults already located in the bile ducts), the clinical manifestations are associated with obstruction of the bile ducts. It can also produce systemic manifestations of the liver, gallbladder and pancreas inflammation also as nauseas, vomits, dyspepsia or even jaundice (3).
The reports of acute hepatic infection describe hepatic abscesses, with the most frequent location being subcapsular (4-6). The other reports of infection of the bile duct are related to obstruction of the bile duct by the adult parasite, producing cases of obstructive jaundice and cholangitis (7-10). There are reports of extrahepatic location (spleen, peritoneum) produced either by migration of the parasite or the immune response at the expense of eosinophils (11,12).
There are reports of parasitic infection clinically simulating intrahepatic cholangiocarcinoma, but not with the imaging and anatomopathological findings of this case (13,14).
In this case, when the patient was questioned again, there was no history of ingesting short-stem vegetables like watercress or lettuce that might be infected. In a recent Chilean report, it was found that every patient with acute fascioliasis in the series (four in total) had a history of raw watercress consumption (15).
The clinical presentation is often vague and unspecific, with a varied group of symptoms due to the acute and chronic phases. In this case in particular the patient had no history of fever or jaundice; and her reason for consultation was only abdominal pain. In addition, the laboratory examinations indicated no parameters of inflammation or eosinophilia that could make one suspect fascioliasis. On this point it should be emphasized that the eosinophilia rate in the endemic areas is close to 50% (16) and that in a report of four cases in Chile, every one presented eosinophilia (15). It is worthy of note that in the pathology study of our case, only Fasciola hepatica eggs were found, which shows the infestation occurred several weeks before, time in which the larva migrate to the bile duct and mature until laying eggs, which is why a chronic stage of fascioliasis should be considered.
All the imaging examinations of this case were consistent with the diagnosis of intrahepatic cholangiocarcinoma. Although the tumor markers were negative, we must remember that their utility in the diagnosis of intrahepatic cholangiocarcinoma is low (16).
There are two reports with hepatic inflammatory pseudotumor in which Ca 19-9 was elevated in contrast with our case (17).
Due to the complexity of the location and the lack of sensitivity of the preoperative biopsy, some authors do not recommend the histopathological study before referring the patient to a hepatic resection when there is suspicion of intrahepatic cholangiocarcinoma (18).
In terms of the benign lesions an intrahepatic cholangiocarcinoma can simulate, these include inflammatory pseudotumors, parasitic infections and some rare varieties of hepatic hemangiomas (19-22).
The imaging findings in the acute phase of hepatic fascioliasis may correspond in CT to multiple hypodense, small, round, oval lesions or in a cluster, with peripheral uptake of the contrast medium. Hypodense nodular lesions arise in the subcapsular area in the first weeks after ingestion of the parasites, progressing to confluent lesions over the following six weeks. Less than 10% of cases present with a single hepatic lesion (23,24). In addition, the CT can reveal subcapsular low attenuation regions in the liver, focal thickening and enhancement of the capsule secondary to the penetration of the parasites in Glisson’s capsule and in some cases hemorrhagic foci (25,26). A T2-weighted MR image shows capsular hyperintensity in the area where the parasite has penetrated. The migration paths can appear initially hypointense and hyperintense in the subcapsular region in T1- and T2-weighted sequences, respectively. Parenchymatous lesions can be seen with the same signal intensity and peripheral enhancement with contrast medium (26). In chronic phase the extent of the parenchymatous lesions quickly disappear after the eighth week post-infection. The CT shows dilated bile ducts, and a progressive decrease in periportal attenuation around the tenth week post-infection (23,27). The MRI can best show ductal dilatation. Capsular or subcapsular fibrotic scars can also be seen during this phase, with heterogeneous signal intensity on the MR images. Sometimes adult trematodes can be identified as a filling defect in the dilated ducts (28). It is also possible to see adenopathies in the hepatic hilum (24). This case, unlike what has been reported in most publications, appeared as a single mass, with progressive enhancement with the contrast medium, with no subcapsular alterations, simulating the behavior of a cholangiocarcinoma. Most cholangiocarcinomas have abundant fibrous stroma and in CT and MRI with dynamic contrast present an initial ring-like enhancement and a progressive, delayed and persistent enhancement of the tumor. These imaging findings are thought to be due to the abundant fibrous content and to a slow diffusion of the contrast material in the tumor interstitium (29,30). The morphological and growth patterns of cholangiocarcinomas described by the Japanese Liver Cancer Group include mass-forming, periductal-infiltrating, intraductal or mixed (31); the first is the most similar to our case. Some cases have been reported as fascioliasis simulating cholangiocarcinoma in MRI, but only as a filling defect of the bile duct and not as a hepatic mass (17,18,32). To our knowledge, there are no reports in which a hepatic fascioliasis appears on imaging simulating peripheral intrahepatic cholangiocarcinoma in CT and MRI. One distinctive finding may be the capsular retraction, which is described in cholangiocarcinoma, but not in fascioliasis (29).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
References
- Mas-Coma S, Valero MA, Bargues MD. Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol 2009;69:41-146. [PubMed]
- Alcaíno H, Apt W. Algunos antecedentes sobre la fasciolosis animal y humana. Monog Med Vet 1989;11:14-29.
- Marcos Raymundo LA, Maco Flores V, Terashima Iwashita A, et al. Clinical characteristics of chronic infection by Fasciola hepatica in children. Rev Gastroenterol Peru 2002;22:228-33. [PubMed]
- Kim TY, Lee YS, Yun JH, et al. A case of probable mixed-infection with Clonorchis sinensis and Fasciola sp.: CT and parasitological findings. Korean J Parasitol 2010;48:157-60. [PubMed]
- Das K, Sakuja P, Aggarwal A, et al. Non-resolving liver abscess with Echinococcus cross-reactivity in a non-endemic region. Indian J Gastroenterol 2007;26:92-3. [PubMed]
- Perry W, Goldsmid JM, Gelfand M. Human fascioliasis in Rhodesia. Report of a case with a liver abscess. J Trop Med Hyg 1972;75:221-3. [PubMed]
- Miranda M, Martínez F. Fascioliasis hepatica causing bile duct obstruction. Rev Med Chil 1988;116:1186-90. [PubMed]
- Banna P, Gulisano G, Musco A, et al. Obstruction of the principal bile duct caused by Fasciola hepatica. First case in Sicily. Minerva Med 1980;71:2555-64. [PubMed]
- Belgraier AH. Common bile duct obstruction due to Fasciola hepatica. N Y State J Med 1976;76:936-7. [PubMed]
- Nicholas JL. Obstruction of the common bile-duct by Fasciola hepatica. Occurrence in a boy of 12 years. Br J Surg 1970;57:544-6. [PubMed]
- Zali MR, Ghaziani T, Shahraz S, et al. Liver, spleen, pancreas and kidney involvement by human fascioliasis: imaging findings. BMC Gastroenterol 2004;4:15. [PubMed]
- Vercelli-Retta J, Lagios MD, Chandrasoma P. Fasciola hepatica and parasitic eosinophilic granuloma of the Liver. Am J Surg Pathol 2002;26:1238. [PubMed]
- Kim YH, Kang KJ, Kwon JH. Four cases of hepatic fascioliasis mimicking cholangiocarcinoma. Korean J Hepatol 2005;11:169-75. [PubMed]
- Yalav O, Yağmur Ö, Ülkü A, et al. A rare cause of obstructive jaundice: Fasciola hepatica mimicking cholangiocarcinoma. Turk J Gastroenterol 2012;23:604-7. [PubMed]
- Fica A, Dabanch J, Farias C, et al. Acute fascioliasis--clinical and epidemiological features of four patients in Chile. Clin Microbiol Infect 2012;18:91-6. [PubMed]
- Dhanasekaran R, Hemming AW, Zendejas I, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep 2013;29:1259-67. [PubMed]
- Ogawa T, Yokoi H, Kawarada Y. A case of inflammatory pseudotumor of the liver causing elevated serum CA19-9 levels. Am J Gastroenterol 1998;93:2551-5. [PubMed]
- Buc E, Lesurtel M, Belghiti J. Is preoperative histological diagnosis necessary before referral to major surgery for cholangiocarcinoma? HPB (Oxford) 2008;10:98-105. [PubMed]
- Inaba K, Suzuki S, Yokoi Y, et al. Hepatic inflammatory pseudotumor mimicking intrahepatic cholangiocarcinoma: report of a case. Surg Today 2003;33:714-7. [PubMed]
- Kitajima K, Shiba H, Nojiri T, et al. Intrahepatic cholangiocarcinoma mimicking hepatic inflammatory pseudotumor. J Gastrointest Surg 2007;11:398-402. [PubMed]
- Park SM, Shin SM, Seo HE, et al. A case of sclerosed hemangioma mimicking intrahepatic cholangiocarcinoma. Korean J Gastroenterol 2009;54:399-403. [PubMed]
- Kim BG, Kang DH, Choi CW, et al. A case of clonorchiasis with focal intrahepatic duct dilatation mimicking an intrahepatic cholangiocarcinoma. Clin Endosc 2011;44:55-8. [PubMed]
- Gonzalo-Orden M, Millán L, Alvarez M, et al. Diagnostic imaging in sheep hepatic fascioliasis: ultrasound, computer tomography and magnetic resonance findings. Parasitol Res 2003;90:359-64. [PubMed]
- Kabaalioglu A, Ceken K, Alimoglu E, et al. Hepatobiliary fascioliasis: sonographic and CT findings in 87 patients during the initial phase and long-term follow-up. AJR Am J Roentgenol 2007;189:824-8. [PubMed]
- Kaya M, Beştaş R, Cetin S. Clinical presentation and management of Fasciola hepatica infection: single-center experience. World J Gastroenterol 2011;17:4899-904. [PubMed]
- Koç Z, Ulusan S, Tokmak N. Hepatobiliary fascioliasis: imaging characteristics with a new finding. Diagn Interv Radiol 2009;15:247-51. [PubMed]
- Lim JH, Mairiang E, Ahn GH. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom Imaging 2008;33:157-65. [PubMed]
- Cantisani V, Cantisani C, Mortelé K, et al. Diagnostic imaging in the study of human hepatobiliary fascioliasis. Radiol Med 2010;115:83-92. [PubMed]
- Menias CO, Surabhi VR, Prasad SR, et al. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics 2008;28:1115-29. [PubMed]
- Sainani NI, Catalano OA, Holalkere NS, et al. Cholangiocarcinoma: current and novel imaging techniques. Radiographics 2008;28:1263-87. [PubMed]
- Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288-91. [PubMed]
- Mohammad Alizadeh AH, Roshani M, Lahmi F, et al. Cholangiocarcinoma in magnetic resonance cholangiopancreatography and fascioliasis in endoscopic ultrasonography. Case Rep Gastroenterol 2011;5:569-77. [PubMed]


