Hepatitis B virus large surface protein: function and fame
Introduction
Chronic infection with hepatitis B virus (HBV) affects 350 to 400 million individuals worldwide and is the leading cause of liver cirrhosis and hepatocellular carcinoma (HCC) worldwide. More than 780,000 people die every year due to the consequences of hepatitis B (WHO 2014). Although much is known about HBV structure and replication cycle (1) the pathogenic mechanisms responsible for liver injury, cirrhosis development and malignant transformation during chronic HBV infection are not well understood. The inability to achieve a complete cure of chronic HBV infections is among the problems that still remain today.
Hepatitis B virus (HBV)
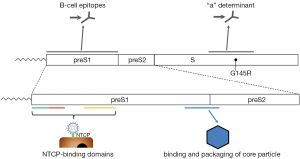
Hepatitis B was recognized as a disease in ancient times, but the virus itself was identified not until 1965 (2). HBV is one of the smallest enveloped DNA viruses and the prototype member of the family of Hepadnaviridae. Infectious HBV has a spherical structure consisting of hepatitis B surface antigen (HBsAg) that envelops the viral nucleocapside, which is formed by the core protein (HBcAg). The encapsidated viral genome is organized as a relaxed circular partially double-stranded DNA (rcDNA) (3). Upon infection of hepatocytes, the HBV rcDNA is converted by cellular enzymes into a covalently closed circular DNA (cccDNA) inside nuclei of infected cells. Episomal HBV cccDNA persists in the hepatocyte as a stable minichromosome organized by histone and non-histone proteins. The viral minichromosome utilizes the cellular transcriptional machinery to produce all viral RNAs necessary for protein production and viral replication (4). The HBV genome contains four overlapping open-reading frames that encode the viral polymerase, HBV surface proteins, the structural core protein and the non-structural precore protein, also known as secreted e-antigen (HBeAg), and the X protein (1). HBV surface (HBs) proteins—the large (LHBs), middle (MHBs), and small (SHBs) can be distinguished by their different domains and glycosylation status (1). The carboxyterminal domain containing SHBs is present in all surface-proteins, preS1 N-terminal extension only in LHBs, preS2 in LHBs and MHBs (5) (Figure 1). These three forms of HBV surface proteins represent HBsAg (2). Surface protein synthesis occurs in endoplasmic reticulum (ER) and could lead to the situation that amounts of protein produced exceed those required for virion assembly. Excess surface proteins undergo multimerization resulting in their budding from the ER/Golgi compartment as both non-infectious spherical and filamentous subviral particles (SVP) or as virions (3). The SVPs typically outnumber the virions, could be components of circulating immune complexes (7), and may induce immune tolerance by a mechanism of “viral apoptotic-like mimicry” (8).
HBV receptor: sodium taurocholate cotransporting polypeptide (NTCP)
PreS extensions of LHBs and MHBs possess certain features that are very important for HBV infectivity such as binding the nucleocapsid during virus envelopment (3) as well as receptor recognition and binding (9,10). The best characterized determinant is a myristoylated motif within the N-terminal preS1 domain where myristoylation-deficient mutants assemble but are non-infectious (11,12). A mimic of preS1 peptide (Myrcludex B®) that inhibits HBV entry in hepatocytes is currently being evaluated in clinical trials for anti-viral activity in chronic HBV-infected patients (13). HBV binding and entry starts with a low-specific binding to heparan-sulfate proteoglycans (14-16), followed by binding to high-specific receptor that was unidentified recently (Figure 1) (17). Using HBV-susceptible primary hepatocytes from Asian tree shrews (Tupaia belangeri) in a biochemical approach, the authors detected NTCP as a binding factor for HBV preS1 peptides, and HBV or HDV (17). The identification of NTCP as a bona fide receptor has revealed a suitable target for HBV entry inhibition. NTCP receptor function is blocked by a variety of different agents including Myrcludex B®, a synthetic N-acylated preS1-derived lipopeptide that inhibits HBV entry in vitro and in vivo with high efficacy (18). Entry inhibitors in combination with nucleos(t)ide analogues could block re-infection and shield naive hepatocytes that emerge from natural liver turnover, opening up new therapeutic options.
Clinical relevance of HBsAg
Primary treatment goals for patients with HBV infection are to prevent progression of the disease, particularly to cirrhosis, liver failure, and HCC (19). The clinical relevance of HBsAg levels is inferred from the relationship of this marker to the intrahepatic amount of cccDNA. There is a correlation between serum HBsAg concentrations and the intrahepatic levels of cccDNA, with the highest levels occurring in HBeAg positive hepatitis B and the lowest in patients with resolved hepatitis (20-23). Through this association, the amount of circulating HBsAg is thought to indirectly measure the control of infection by the immunological response independent from the antiviral response, which can be assessed by measuring HBV DNA levels in serum. HBsAg titres allow to monitor the natural history of HBV infection and to predict treatment outcome. HBsAg quantification can be used to differentiate between inactive carriers and HBeAg-negative chronic hepatitis B patients, who are likely to reactivate HBV infection and who can benefit from therapy (24). Early HBsAg monitoring could be also used during PEG-IFN therapy to develop a response-guided algorithm to stop or switch therapy at week 12 in poor responders, to continue standard 48-week treatment in most patients with a favourable response, and to extend therapy for intermediate on-treatment responders to improve the chances of response (25). The addition of entecavir (ETV) or tenofovir disoproxil fumarate (TDF) to PEG-IFN therapy has been shown to increase on treatment HBsAg decline, HBeAg seroconvertion and sustained virological response rate (26-28). Several studies suggest that baseline treatment and on-treatment HBsAg levels might help identify patients, who can stop therapy with a reduced risk of reactivation (25). In summary, analysis of serum HBsAg level is a non-invasive diagnostic parameter that improves HBV treatment opportunities.
It is recognized that genomic hyper-variability allows HBV to escape selection pressures imposed by antiviral therapies, vaccines and the host immune system (29,30). There is an increasing prevalence of mutant large surface antigens in serum. Although mutations occur throughout the HBV genome, they tend to cluster into mutational patterns in particular, the basal core promoter, the pre-core region, the polymerase gene and the “a” determinant of HBsAg (31) (Figure 1). Because HBsAg mutants with altered antigenicity most frequently variants selected under active or passive immunoprophylaxis or antiviral treatments could be viable and pathogenic, their spread would have substantial consequences for public health (6).
HBV-modulated immune response
T cell exhaustion is a state of T cell dysfunction (Figure 2) that arises during many chronic infections and cancer. It is defined by poor effector function, sustained expression of inhibitory receptors, and a transcriptional state distinct from that of functional effector or memory T cells (32). A particular characteristic of chronic HBV infection is the production of large quantities of SVPs containing only surface antigens and associated host-derived lipid (33). After long exposure to high levels of viral antigen in chronic hepatitis B patients, T cells either display an exhausted phenotype (34-37) or are deleted (38,39). SVPs are non-infectious and their enhanced production could be a way of subverting the antiviral response (40,41).
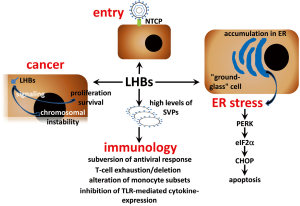
Peripheral blood monocytes (PBMC) supply peripheral tissues with macrophage and dendritic cell (DC) precursors and, in the setting of infection, also contribute directly to immune defence against microbial pathogens (42). Chronic HBV infection was shown to alter monocyte subsets frequencies depending on the clinical phase (43,44). HBV does not infect or productively replicate in human PBMCs (45). However, HBsAg particles are highly immunogenic, and DCs and macrophages from mice cross-present recombinant HBsAg particles to CD8+ T cells in the absence of inflammatory signals (46-48). These studies have been performed in mice or in vitro model systems and demonstrated that HBV antigens have the ability to activate HBV-specific CD8+ T cells, which play a key role in HBV control (49). However, there is controversy on the effect of HBsAg on monocytes and DCs from chronic HBV-infected patients. It was reported that HBV has an inhibitory effect on TLR-mediated cytokine production by antigen-presenting cells (APC) and could affect innate immunity pathways (50-53). In contrast, other studies did not demonstrate any significant alterations in T cell stimulatory function of different APCs (54,55). More interesting, it was recently shown that CD14-positive monocytes retained an HBsAg depot and differentiation of HBsAg+ CD14 monocytes from chronic patients to DCs induced cross-presentation of the intracellular reservoir of viral antigen. Thus, circulating HBsAg selectively captured by monocytes can be used advantage as a personalized antigenic reservoir to activate virus-specific T cells in chronic HBV patients (54).
Growing number of HBV mutants and recently discovered mechanisms of virus-mediated immune response modulation forced to look for new ways for immunoprophylaxis of HBV infection. Promising concepts for preventive and therapeutic vaccination are summarized in Table 1.
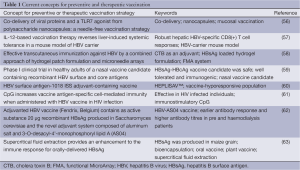
Full table
Direct cytopathic effects of HBs
It is widely accepted that these events originate from persistent immune pathogenesis (15,64), but observations in patients with chronic hepatitis B under conditions of immune suppression and in transgenic mouse models of HBV infection suggest, that in absence of adaptive immune responses cellular mechanisms induced by HBV may also lead to the development of these liver diseases (65-68). Chronic HBV carriers after administration of immunosuppressive drugs for an autoimmune disorder (69) or after liver (70-72), renal (73,74), or bone marrow (75) transplantation demonstrated increased viremia accompanied by high viral protein expression in infected hepatocytes. Some of these patients developed fibrosing cholestatic hepatitis (FCH), an aggressive and mostly fatal form of viral hepatitis. FCH is associated with increased viral replication (76) and is characterized by high intrahepatic expression of viral proteins, diffuse hepatocyte ballooning, the presence of ground-glass hepatocytes, prominent cholestasis, and periportal fibrosis (70-73,76,77). To examine whether HBV has direct cytopathic effects in immune compromised hosts, immune deficient mice (uPA-SCID) harboring human liver cells were infected with HBV. Histological analysis of the livers of long-term-infected uPA-SCID chimeras showed, that the majority of human hepatocytes had a ground-glass appearance, stained intensely for viral proteins, and showed signs of considerable damage and cell death (67). This histopathologic pattern closely resembles the picture observed in the livers of other transgenic mice that expressed HBV surface proteins (67,78-81). A relative increase in production of LHBs compared with that of SHBs (HBsAg) led to profound reduction of the HBsAg concentration in serum as a result of accumulation of both surface polypeptides in a relatively insoluble compartment within the cell (78). Furthermore, overproduction of LHBs resulted in the formation of extremely long (up to 800 nm), occasionally branching, filamentous 22-nm-diameter HBsAg particles, that accumulate within the ER of the hepatocyte and are not efficiently secreted. The hepatocytes become enlarged, hydropic, and eosinophilic and also display the characteristic features of “ground-glass” cells (Figure 2). As filament storage progresses, the ground-glass cells undergo coagulative necrosis and the mice develop an age-dependent lesion, whose severity is related to the intracellular concentration of surface proteins, that is characterized by focal hepatocellular degeneration and necrosis, lobular macrophagic inflammation, and increased serum transaminase activity. Thus, progressive intracellular accumulation of HBsAg, which can reach sufficiently high concentrations, could be directly cytotoxic to hepatocytes in this transgenic mouse system (79). Prolonged hepatocellular injury in these mice initiated a programmed response within the liver, characterized by inflammation, regenerative hyperplasia, transcriptional deregulation, and aneuploidy progressing to neoplasia. The incidence of HCC in this model corresponded to the frequency, severity, and age of onset of liver cell injury, which itself corresponds to the intrahepatic concentration of HBsAg and is influenced by genetic background and sex (80,81). Moreover, these mice were largely tolerant to the transgenic products (82) suggesting that the mouse expressing HBV surface proteins in the liver could be an excellent model for investigation of their direct cytotoxic effects. Since hepatic fibrosis constitutes the wound healing response to liver injury (83) this transgenic mouse-model began recently to draw an attention as model for investigation of liver fibrosis (84,85). Development of hepatic fibrosis after chemical liver injury is enhanced in BALB/c mice exhibiting a Th2 response compared to C57BL/6 mice, which demonstrated a primary Th1 response (86,87). As most studies were performed using HBV surface proteins expressing mice on fibrosis-resistant C57BL/6 genetic background, we backcrossed these mice to fibrosis-susceptible BALB/c genetic background (81). Despite the same level of HBV surface protein hepatic expression in transgenic mice on both genetic background comparative biochemical and immunohistochemical analysis revealed remarkable differences in liver pathogenesis between these two mouse strains. As expected we observed enhanced liver fibrosis in BALB/c mice that was a consequence of stronger liver injury (81). Previously it was demonstrated that over-expression of LHBs in human hepatoma cells (88) and accumulation of HBV pre-S mutants in the ER of transgenic mice hepatocytes resulted in induction of ER stress (89). As a response to ER stress induction by accumulation of misfolded proteins the unfolded protein response (UPR) is activated. Distinct branches of UPR are mediated by three different classes of ER-membrane transducers: inositol-requiring protein-1 (IRE1), activating transcription factor-6 (ATF6) or protein kinase-like endoplasmic reticulum kinase (PERK). PERK activation causes the phosphorylation of the alpha subunit of eukaryotic translation-initiation factor 2α (eIF2α) (90-92). From three UPR branches only PERK was activated in the liver of transgenic mice on both genetic backgrounds (81). However, the expression of the C/EBP homologous protein (CHOP), also known as growth arrest and DNA damage-inducible gene (GADD) 153, one of the downstream effectors of PERK pathway that mediates pro-apoptotic pathways emanating from the stressed ER (90,92) was strongly induced only in the liver of BALB/c transgenic mice. Thus, the response of the host to the expression of HBV surface proteins depends on its genetic background (81). Furthermore, two branches of UPR IRE1α and ATF6 were not activated in the liver of HBV transgenic mice. PERK branch activation is largely sustained with unmitigated ER stress, whereas persistent ER stress attenuates IRE1α and ATF6 signaling (93). Therefore, permanent expression of HBV surface proteins leads to the activation of persistent ER stress in hepatocytes that induces PERK and impairs another branches of UPR. It is possible that this situation is common for chronic liver disease comprising ER stress induction. ER storage diseases (ERSDs) are a group of genetically based disorders in which mutant proteins fail to pass the ER quality control. ERSD may be caused indirectly by toxic effects of the misfolded protein or aggregates thereof on the cell. Additionally, the cell’s reaction to the ER stress may include signaling pathways, which are ultimately detrimental (94,95). As transgenic mice expressing HBV surface proteins fit all of these criteria, these mice could be excellent model for study ERSDs.
Carcinogenesis
Transgenic mice, that specifically overproduce LHBs in the liver, develop liver tumours (80). Initially, the development of HCC was considered to be caused by a permanent inflammatory process under conditions of LHBs overproduction (79). However, it was shown that LHBs is able to activate nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1). This process is mediated by protein kinase C (PKC)-dependent activation of the c-Raf/MAP-kinase signal transduction pathway triggering cellular proliferation control (96). It is possible that continuous activation of these enzymes might additionally contribute to the development of HCC. The presence of several types of mutants in different replicative stages of chronic HBV infection suggests the potential evolution of virus under immune pressure during HBV infection. Naturally occurring preS mutants are detected frequently in serum obtained from patients with chronic HBV infection. The resulting mutants reveal shorter forms of LHBs proteins with internal deletions. Moreover, preS mutants were identified in ground-glass hepatocytes (GGH) representing a histological hallmark of chronic HBV infection (89). Two different types of GGHs have been defined to be associated with different stages of chronic HBV infection. Type I GGHs containing preS1 mutants are typical for the high viral replicative phase of chronic HBV infection, whereas type II GGHs harboring preS2 mutants are specific for the advanced stages of chronic liver disease (89,97). Furthermore, HBV preS mutants, especially preS2, were associated with an increased risk of HCC (97,98). It has been observed, that the ER stress response induced by preS mutated proteins is responsible for the enhanced expression of vascular endothelial growth factor-A (VEGF-A) and for the activation of Akt/mammalian target of rapamycin (Akt/mTOR) signaling in GGHs (99). In addition, it has been shown that preS2 mutated proteins may directly interact with the Jun activation domain-binding protein 1 (JAB1), thus triggering cyclin-dependent kinase (Cdk) inhibitor p27 degradation, Retinoblastoma hyperphosphorylation and cell cycle progression (100). The preS2 mutants may also induce the overexpression of both, cyclin A and cyclooxygenase-2, thereby leading to cell cycle progression, cell proliferation, and anchorage-independent growth. Furthermore, in livers of preS2 mutant-transgenic mice it has been shown, that cyclin A is located in the cytoplasm rather than in the nucleus. This aberrantly expressed form of cyclin A is implicated in centrosome over-duplication, which represents a potential mechanism for chromosome instability (6,101). Thus, carriers of preS mutants represent a high risk group of chronic HBV infected patients for HCC development. Specific therapeutic treatment should be applied to these patients to prevent development of HCC. In addition there is a potential association of HBsAg with HCC among patients with chronic HBV infection.
Conclusions
HBV is the leading cause of liver cirrhosis and HCC worldwide. In spite of current treatments a complete cure of chronic HBV infections remains a challenge. We have discussed multiple pathological aspects of HBV surface antigens. HBV large surface antigen is becoming more important because of the identification of HBV receptor, the correlation of HBsAg levels with cccDNA in livers, the significance of HBV pre-S mutants in anti-viral treatment and HCC development, the exclusive identification of pre-S mutants in ground glass hepatocytes and the prediction of HCC development. In addition hepatitis B surface proteins could participate to ER storage diseases. An association between HBV preS mutated proteins and increased risk of HCC has been detected recently. Taken together, the multifaceted pathological aspects of the HBsAg predetermine the design of new therapeutical options modulating associated biological implications.
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (RO 957/8-1), by the faculty of medicine Justus-Liebig-University Giessen, and the von Behring Röntgen Foundation.
Disclosure: The authors declare no conflict of interest.
References
- Glebe D, Bremer CM. The molecular virology of hepatitis B virus. Semin Liver Dis 2013;33:103-12. [PubMed]
- Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J 2013;10:239. [PubMed]
- Bruss V. Hepatitis B virus morphogenesis. World J Gastroenterol 2007;13:65-73. [PubMed]
- Levrero M, Pollicino T, Petersen J, et al. Control of cccDNA function in hepatitis B virus infection. J Hepatol 2009;51:581-92. [PubMed]
- Glebe D, Urban S. Viral and cellular determinants involved in hepadnaviral entry. World J Gastroenterol 2007;13:22-38. [PubMed]
- Pollicino T, Cacciola I, Saffioti F, et al. Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. J Hepatol 2014;61:408-17. [PubMed]
- Madalinski K, Burczynska B, Heermann KH, et al. Analysis of viral proteins in circulating immune complexes from chronic carriers of hepatitis B virus. Clin Exp Immunol 1991;84:493-500. [PubMed]
- Vanlandschoot P, Leroux-Roels G. Viral apoptotic mimicry: an immune evasion strategy developed by the hepatitis B virus? Trends Immunol 2003;24:144-7. [PubMed]
- Glebe D, Urban S, Knoop EV, et al. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes. Gastroenterology 2005;129:234-45. [PubMed]
- Meier A, Mehrle S, Weiss TS, et al. Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes. Hepatology 2013;58:31-42. [PubMed]
- Bruss V, Hagelstein J, Gerhardt E, et al. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology 1996;218:396-9. [PubMed]
- Gripon P, Le Seyec J, Rumin S, et al. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology 1995;213:292-9. [PubMed]
- Lütgehetmann M, Mancke LV, Volz T, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology 2012;55:685-94. [PubMed]
- Leistner CM, Gruen-Bernhard S, Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol 2008;10:122-33. [PubMed]
- Schuch A, Hoh A, Thimme R. The role of natural killer cells and CD8(+) T cells in hepatitis B virus infection. Front Immunol 2014;5:258. [PubMed]
- Sureau C, Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology 2013;57:985-94. [PubMed]
- Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012;1:e00049. [PubMed]
- Lempp FA, Urban S. Inhibitors of hepatitis B virus attachment and entry. Intervirology 2014;57:151-7. [PubMed]
- Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med 2009;150:104-10. [PubMed]
- Jaroszewicz J, Calle Serrano B, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol 2010;52:514-22. [PubMed]
- Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol 2010;52:508-13. [PubMed]
- Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004;126:1750-8. [PubMed]
- Höner Zu Siederdissen C, Cornberg M. The role of HBsAg levels in the current management of chronic HBV infection. Ann Gastroenterol 2014;27:105-12. [PubMed]
- Martinot-Peignoux M, Lapalus M, Laouénan C, et al. Prediction of disease reactivation in asymptomatic hepatitis B e antigen-negative chronic hepatitis B patients using baseline serum measurements of HBsAg and HBV-DNA. J Clin Virol 2013;58:401-7. [PubMed]
- Martinot-Peignoux M, Lapalus M, Asselah T, et al. HBsAg quantification: useful for monitoring natural history and treatment outcome. Liver Int 2014;34 Suppl 1:97-107. [PubMed]
- Marcellin P, Martinot-Peignoux M, Lapalus M, et al. Predictive value of qHBsAg for SVR and HBsAg loss in chronic hepatitis B patients receiving pegylated inteferon with or without teneofovir. Hepatology 2013;58:681A.
- Sonneveld MJ, Xie Q, Zhang NP, et al. Adding peginterferon alfa-2a to entecavirincreases HBsAg decline and HBeAg clearance - first results from a global randomized trial (ARES study). Hepatology 2012;56:199A.
- Takkenberg RB, Jansen L, de Niet A, et al. Baseline hepatitis B surface antigen (HBsAg) as predictor of sustained HBsAg loss in chronic hepatitis B patients treated with pegylated interferon-α2a and adefovir. Antivir Ther 2013;18:895-904. [PubMed]
- Gerlich WH, Glebe D, Schüttler CG. Deficiencies in the standardization and sensitivity of diagnostic tests for hepatitis B virus. J Viral Hepat 2007;14 Suppl 1:16-21. [PubMed]
- Hollinger FB. Hepatitis B virus genetic diversity and its impact on diagnostic assays. J Viral Hepat 2007;14 Suppl 1:11-5. [PubMed]
- Weber B. Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact. J Clin Virol 2005;32:102-12. [PubMed]
- Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492-9. [PubMed]
- Millman I, Zavatone V, Gerstley BJ, et al. Australia antigen detected in the nuclei of liver cells of patients with viral hepatitis by the fluorescent antibody technic. Nature 1969;222:181-4. [PubMed]
- Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215-25. [PubMed]
- Das A, Hoare M, Davies N, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 2008;205:2111-24. [PubMed]
- Lopes AR, Kellam P, Das A, et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest 2008;118:1835-45. [PubMed]
- Schurich A, Khanna P, Lopes AR, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011;53:1494-503. [PubMed]
- Maini MK, Boni C, Lee CK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000;191:1269-80. [PubMed]
- Webster GJ, Reignat S, Brown D, et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol 2004;78:5707-19. [PubMed]
- Reignat S, Webster GJ, Brown D, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med 2002;195:1089-101. [PubMed]
- Sette AD, Oseroff C, Sidney J, et al. Overcoming T cell tolerance to the hepatitis B virus surface antigen in hepatitis B virus-transgenic mice. J Immunol 2001;166:1389-97. [PubMed]
- Serbina NV, Jia T, Hohl TM, et al. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008;26:421-52. [PubMed]
- Anthony DD, Umbleja T, Aberg JA, et al. Lower peripheral blood CD14+ monocyte frequency and higher CD34+ progenitor cell frequency are associated with HBV vaccine induced response in HIV infected individuals. Vaccine 2011;29:3558-63. [PubMed]
- Zhang JY, Zou ZS, Huang A, et al. Hyper-activated pro-inflammatory CD16 monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B. PLoS One 2011;6:e17484. [PubMed]
- Untergasser A, Zedler U, Langenkamp A, et al. Dendritic cells take up viral antigens but do not support the early steps of hepatitis B virus infection. Hepatology 2006;43:539-47. [PubMed]
- Böhm W, Schirmbeck R, Elbe A, et al. Exogenous hepatitis B surface antigen particles processed by dendritic cells or macrophages prime murine MHC class I-restricted cytotoxic T lymphocytes in vivo. J Immunol 1995;155:3313-21. [PubMed]
- Schirmbeck R, Böhm W, Melber K, et al. Processing of exogenous heat-aggregated (denatured) and particulate (native) hepatitis B surface antigen for class I-restricted epitope presentation. J Immunol 1995;155:4676-84. [PubMed]
- Boltjes A, Groothuismink ZM, van Oord GW, et al. Monocytes from chronic HBV patients react in vitro to HBsAg and TLR by producing cytokines irrespective of stage of disease. PLoS One 2014;9:e97006. [PubMed]
- Thimme R, Wieland S, Steiger C, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77:68-76. [PubMed]
- Op den Brouw ML, Binda RS, van Roosmalen MH, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology 2009;126:280-9. [PubMed]
- van der Molen RG, Sprengers D, Binda RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology 2004;40:738-46. [PubMed]
- Woltman AM, Op den Brouw ML, Biesta PJ, et al. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One 2011;6:e15324. [PubMed]
- Wu J, Meng Z, Jiang M, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 2009;49:1132-40. [PubMed]
- Gehring AJ, Haniffa M, Kennedy PT, et al. Mobilizing monocytes to cross-present circulating viral antigen in chronic infection. J Clin Invest 2013;123:3766-76. [PubMed]
- Tavakoli S, Mederacke I, Herzog-Hauff S, et al. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clin Exp Immunol 2008;151:61-70. [PubMed]
- Vicente S, Peleteiro M, Díaz-Freitas B, et al. Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: a needle-free vaccination strategy. J Control Release 2013;172:773-81. [PubMed]
- Zeng Z, Kong X, Li F, et al. IL-12-based vaccination therapy reverses liver-induced systemic tolerance in a mouse model of hepatitis B virus carrier. J Immunol 2013;191:4184-93. [PubMed]
- Guo L, Qiu Y, Chen J, et al. Effective transcutaneous immunization against hepatitis B virus by a combined approach of hydrogel patch formulation and microneedle arrays. Biomed Microdevices 2013;15:1077-85. [PubMed]
- Betancourt AA, Delgado CA, Estévez ZC, et al. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int J Infect Dis 2007;11:394-401. [PubMed]
- Cooper C, Mackie D. Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: a review of HEPLISAV™ safety and efficacy. Expert Rev Vaccines 2011;10:417-27. [PubMed]
- Angel JB, Cooper CL, Clinch J, et al. CpG increases vaccine antigen-specific cell-mediated immunity when administered with hepatitis B vaccine in HIV infection. J Immune Based Ther Vaccines 2008;6:4. [PubMed]
- Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin Biol Ther 2008;8:235-47. [PubMed]
- Hayden CA, Smith EM, Turner DD, et al. Supercritical fluid extraction provides an enhancement to the immune response for orally-delivered hepatitis B surface antigen. Vaccine 2014;32:1240-6. [PubMed]
- Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010;58:258-66. [PubMed]
- Pol S. Management of HBV in immunocompromised patients. Liver Int 2013;33 Suppl 1:182-7. [PubMed]
- Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis 2013;33:167-77. [PubMed]
- Meuleman P, Libbrecht L, Wieland S, et al. Immune suppression uncovers endogenous cytopathic effects of the hepatitis B virus. J Virol 2006;80:2797-807. [PubMed]
- Sugiyama M, Tanaka Y, Kurbanov F, et al. Direct cytopathic effects of particular hepatitis B virus genotypes in severe combined immunodeficiency transgenic with urokinase-type plasminogen activator mouse with human hepatocytes. Gastroenterology 2009;136:652-62. [PubMed]
- Zanati SA, Locarnini SA, Dowling JP, et al. Hepatic failure due to fibrosing cholestatic hepatitis in a patient with pre-surface mutant hepatitis B virus and mixed connective tissue disease treated with prednisolone and chloroquine. J Clin Virol 2004;31:53-7. [PubMed]
- Davies SE, Portmann BC, O'Grady JG, et al. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology 1991;13:150-7. [PubMed]
- Lau JY, Bain VG, Davies SE, et al. High-level expression of hepatitis B viral antigens in fibrosing cholestatic hepatitis. Gastroenterology 1992;102:956-62. [PubMed]
- Benner KG, Lee RG, Keeffe EB, et al. Fibrosing cytolytic liver failure secondary to recurrent hepatitis B after liver transplantation. Gastroenterology 1992;103:1307-12. [PubMed]
- Chen CH, Chen PJ, Chu JS, et al. Fibrosing cholestatic hepatitis in a hepatitis B surface antigen carrier after renal transplantation. Gastroenterology 1994;107:1514-8. [PubMed]
- Hung YB, Liang JT, Chu JS, et al. Fulminant hepatic failure in a renal transplant recipient with positive hepatitis B surface antigens: a case report of fibrosing cholestatic hepatitis. Hepatogastroenterology 1995;42:913-8. [PubMed]
- McIvor C, Morton J, Bryant A, et al. Fatal reactivation of precore mutant hepatitis B virus associated with fibrosing cholestatic hepatitis after bone marrow transplantation. Ann Intern Med 1994;121:274-5. [PubMed]
- Mason AL, Wick M, White HM, et al. Increased hepatocyte expression of hepatitis B virus transcription in patients with features of fibrosing cholestatic hepatitis. Gastroenterology 1993;105:237-44. [PubMed]
- Naoumov NV, Portmann BC, Tedder RS, et al. Detection of hepatitis B virus antigens in liver tissue. A relation to viral replication and histology in chronic hepatitis B infection. Gastroenterology 1990;99:1248-53. [PubMed]
- Chisari FV, Filippi P, McLachlan A, et al. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol 1986;60:880-7. [PubMed]
- Chisari FV, Filippi P, Buras J, et al. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci U S A 1987;84:6909-13. [PubMed]
- Chisari FV, Klopchin K, Moriyama T, et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 1989;59:1145-56. [PubMed]
- Churin Y, Roderfeld M, Stiefel J, et al. Pathological impact of hepatitis B virus surface proteins on the liver is associated with the host genetic background. PLoS One 2014;9:e90608. [PubMed]
- Wirth S, Guidotti LG, Ando K, et al. Breaking tolerance leads to autoantibody production but not autoimmune liver disease in hepatitis B virus envelope transgenic mice. J Immunol 1995;154:2504-15. [PubMed]
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425-56. [PubMed]
- Jin Z, Sun R, Wei H, et al. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology 2011;53:219-29. [PubMed]
- Spano D, Cimmino F, Capasso M, et al. Changes of the hepatic proteome in hepatitis B-infected mouse model at early stages of fibrosis. J Proteome Res 2008;7:2642-53. [PubMed]
- Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A 1997;94:10663-8. [PubMed]
- Walkin L, Herrick SE, Summers A, et al. The role of mouse strain differences in the susceptibility to fibrosis: a systematic review. Fibrogenesis Tissue Repair 2013;6:18. [PubMed]
- Xu Z, Jensen G, Yen TS. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J Virol 1997;71:7387-92. [PubMed]
- Wang HC, Huang W, Lai MD, et al. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci 2006;97:683-8. [PubMed]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 2012;13:89-102. [PubMed]
- Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 2011;54:795-809. [PubMed]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519-29. [PubMed]
- Lin JH, Li H, Yasumura D, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007;318:944-9. [PubMed]
- Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev 2007;87:1377-408. [PubMed]
- Rutishauser J, Spiess M. Endoplasmic reticulum storage diseases. Swiss Med Wkly 2002;132:211-22. [PubMed]
- Hildt E, Saher G, Bruss V, et al. The hepatitis B virus large surface protein (LHBs) is a transcriptional activator. Virology 1996;225:235-9. [PubMed]
- Sinn DH, Choi MS, Gwak GY, et al. Pre-s mutation is a significant risk factor for hepatocellular carcinoma development: a long-term retrospective cohort study. Dig Dis Sci 2013;58:751-8. [PubMed]
- Su IJ, Wang LH, Hsieh WC, et al. The emerging role of hepatitis B virus pre-S2 deletion mutant proteins in HBV tumorigenesis. J Biomed Sci 2014;21:98. [PubMed]
- Yang JC, Teng CF, Wu HC, et al. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis. Hepatology 2009;49:1962-71. [PubMed]
- Hsieh YH, Su IJ, Wang HC, et al. Hepatitis B virus pre-S2 mutant surface antigen induces degradation of cyclin-dependent kinase inhibitor p27Kip1 through c-Jun activation domain-binding protein 1. Mol Cancer Res 2007;5:1063-72. [PubMed]
- Wang HC, Chang WT, Chang WW, et al. Hepatitis B virus pre-S2 mutant upregulates cyclin A expression and induces nodular proliferation of hepatocytes. Hepatology 2005;41:761-70. [PubMed]


