Carotenoids and non-alcoholic fatty liver disease
Introduction
The results of recent investigations suggest that dietary interventions may modulate the expression of some genes that may affect many chronic diseases such as cancer, cardiovascular disease, diabetes, hypertension and neurodegenerative diseases. Carotenoids are among the most investigated dietary micronutrients in this regard.
Carotenoids are fat-soluble pigments that give the yellow, red and orange color to fruits and vegetables. Although at least 600 carotenoids were defined in the nature, approximately forty of these are consumed in the human diet. Furthermore, fourteen of these carotenoids and some of their metabolites are identified in blood and tissues (1,2). They can only be synthesized by plants and microorganisms, therefore their sources are mostly from fruits and vegetables. β-carotene, lycopene, α-carotene, β-cryptoxanthin, lutein and zeaxanthin are among the most studied carotenoids (3-5). Carotenoids can be separated into provitamin A carotenoids (e.g., β-carotene α-carotene and β-cryptoxanthin) and non-provitamin A carotenoids (lycopene, lutein and zeaxanthin). These compounds play an important role in human physiology due to their remarkable biological activities such as their effects on oxidative stress and inflammation (3). Carotenoids can act as potent antioxidants and protect the cells against oxidative damage induced by reactive oxygen species (ROS) (4).
Nonalcoholic fatty liver disease (NAFLD) is the broad spectrum of diseases from simple hepatic steatosis to cirrhosis, without excessive alcohol intake and use of steatogenic medication or hereditary disorders. Hepatic steatosis must be proven by imaging or histopathology. According to the NAFLD diagnosis and management guidelines of the American Association for the Study of Liver Diseases (AASLD), nonalcoholic fatty liver (NAFL) is defined as the presence of hepatic steatosis without ballooning degeneration and inflammation in the hepatocytes. Non-alcoholic steatohepatitis (NASH) is defined as the presence of diffuse fatty infiltration in the liver and characterized by ballooning hepatocyte injury and inflammation in the hepatocytes with or without fibrosis (6-8). NAFL is mostly benign and risk of progression to cirrhosis and liver failure is minimal, whereas NASH can lead to cirrhosis, liver failure and hepatocellular carcinoma (HCC) (8,9).
There are limited and inconsistent data regarding the incidence rate of NAFLD. Inconsistency is related to different age, ethnic groups, and geographic groups (8). Although a study from England has shown an incidence of 29 cases per 100,000 person-years (10), Japanese researchers have reported rates of 31 and 86 suspected cases per 1,000 person-years in two different studies (11,12). NAFLD has become the most common cause of chronic liver disease with increasing prevalence and is reported to affect up to 20-40% of the general adult population worldwide (13). Increased prevalence of the disease has nearly doubled from 1988 to 2008 in the US and it is responsible for over 75% of the chronic liver diseases (14,15). The primary reason of the increased rate in the US population is obesity. Together with obesity, it is commonly associated with diabetes mellitus type 2, metabolic syndrome and hyperlipidemia (7,14).
In this paper, we review the underlying mechanisms of NAFLD pathogenesis, including oxidative stress and inflammation, and discuss the potential preventive and therapeutic effects of carotenoids in NAFLD.
NAFLD pathogenesis
The pathogenesis of NAFLD is a complicated process and is not fully understood. Assessment of the hepatic molecular changes, metabolic signaling networks and other pathogenetic factors could be helpful in understanding the process and developing appropriate preventive or therapeutic interventions. In 1998, a model was proposed to describe the pathogenetic process from simple steatosis NAFL to NASH. According to this widely adopted “two hits” pathophysiological model, first hit refers to hepatic fat accumulation and subsequently the second hit is the increased oxidative stress and initiation of lipid peroxidation. Recently, this model gave place to “multiple parallel hits” hypothesis, which has been suggested by Tilg and Moschen, and involved obesity, insulin resistance, oxidative stress, and proinflammatory processes (16).
Obesity is defined as excessive fat accumulation in adipose tissue and especially visceral adipose tissue, which is more important than subcutaneous adipose tissue in terms of NAFLD. Visceral obesity is associated with hepatic steatosis, hepatic inflammation and fibrosis (6,17,18). It has also been associated with increased insulin resistance, metabolic syndrome, lipogenesis, and lipolysis (19,20).
Hepatic fat accumulation, i.e., steatosis, is primarily associated with increased intake of dietary fat and/or increased free fatty acids (FFA) due to increased lipolysis from adipose tissue. Furthermore decreased FFA oxidation, decreased hepatic very low-density lipoprotein (VLDL), triglyceride secretion and increased hepatic lipogenesis contribute to the steatosis (16,21). FFAs are responsible for approximately two-thirds of accumulated lipid in liver (22).
Insulin resistance has been related to metabolic syndrome and type 2 diabetes (23). Hepatic insulin resistance is characterized by hyperinsulinemia, hyperglycemia and an increase in VLDL production. Accordingly the hepatic excessive VLDL leads to a low high-density lipoprotein (HDL) concentration and hypertriglyceridemia (9,24,25).
Oxidative stress and inflammatory process in NAFLD
Oxidative stress refers to an imbalance between the ROS and the antioxidant molecules (26). Oxidative stress stimulated by ROS could generate oxidative damage and lead to chronic diseases. ROS are highly reactive and thus may interact with cellular membranes, proteins and nucleic acids (27,28). Oxidative stress may result from hepatic overloading of FFA which induces mitochondrial β-oxidation or microsomal enzymes such as cytochrome P4502E1 (CYP2E1). Increased CYP-P450 activity has been observed in obesity and NAFLD in both humans and rodents (29). Increased fatty acid oxidation in mitochondria and CYP2E1 have enhanced NADPH oxidase activity resulting in increased production of the ROS superoxide and hydrogen peroxide by redox cycling of endogenous and exogenous substrates (30-32). ROS overproduction causes cellular damage, which induces lipid peroxidation and consequently mitochondrial dysfunction that contributes to hepatocellular damage. In response to the state of oxidative stress, superoxide dismutase, glutathione (GSH) peroxidase, and catalase activities are increased in NAFLD (33).
ROS production has also indirect hepatotoxic effect via mitochondrial β-oxidation. The peroxisome proliferator activated receptors (PPAR) regulate the fatty acid metabolism and storage. FFA-induced PPAR-α up-regulates carnitine palmitoyltransferase-1 (CPT-1) expression and increases the mitochondrial β-oxidation, and regulates the uptake and clearance of fatty acids (14,34,35). Knockout models of PPAR-α have been associated with steatosis, implicating a possible role for PPAR-α in NAFLD (36). Moreover, PPAR-γ is involved in insulin sensitivity and triglyceride storage and is connected with NAFLD. PPAR-γ levels have been found to be increased in the livers of NAFLD mice (14,37).
Tumor necrosis factor-α (TNF-α) and IL-6 are two important pro-inflammatory cytokines produced by injured hepatocytes, immune cells, and activated Kupffer cells and play an important role in inflammation. Both of TNF-α and IL-6 are increased in patients with NASH and their levels are correlated with the severity of inflammation, fibrosis and histological changes in the liver (38-40). Increased TNF-α activates c-Jun N-terminal kinase (JNK) signaling pathway resulting in hepatocyte apoptosis (41).
As mentioned above, NASH is characterized by steatosis with inflammation and hepatocyte ballooning with or without fibrosis (6,8). Leading feature of the NASH is the presence of inflammation and fibrosis. Hepatic stellate cells synthesize several growth factors and extracellular matrix materials such as collagen (42,43). Proliferation of these cells and collagen synthesis induced by cytokines such as TGF-β by Kupffer cells and oxidative stress play a key role in hepatic fibrosis (44,45).
Carotenoids and NAFLD
Due to increasing prevalence and incidence, and lack of established therapeutic intervention, NAFLD has become one of the most important health problems in the world. Obesity and metabolic disorders related to excessive fat intake play an important role in the pathogenesis of NAFLD. Epidemiological studies suggest that lifestyle modifications, such as altered diet with reduced caloric intake, weight loss, and physical activity are safe and effective interventions for improving obesity-mediated insulin resistance and NAFLD (6,46). Therefore, many natural dietary compounds have been studied for prevention and treatment of NAFLD. Carotenoids are among the most studied dietary compounds and their sources are mostly fruits and vegetables. Among the carotenoids, lycopene and β-carotene are the most studied compounds. The exact mechanisms of the protective effects of carotenoids in NAFLD is unclear, but there is evidence from various experimental studies that carotenoids may work through multiple mechanisms, including antioxidant and anti-inflammatory effects (3,4).
In a human study which investigated the association of NAFL and serum levels of carotenoids, serum β-carotene was decreased with fat accumulation in the liver, but the levels of lycopene, α-carotene, β-cryptoxanthin, and lutein were not decreased (47). Another human study reported that plasma levels of carotenoids (lutein, zeaxanthin, β-cryptoxanthin, lycopene, α-carotene, and β-carotene) were significantly decreased in patients with NASH compared to control subjects (48).
Lycopene and NAFLD
Lycopene is a carotenoid that gives tomato and watermelon their red color. It has no vitamin A activity and is classified as a non-provitamin A carotenoid (27,49). Lycopene is a fat-soluble hydrocarbon with 40 carbon atoms and 56 hydrogen atoms (C40H56) (49). It is predominantly concentrated in fatty tissues like adrenal glands, liver, testis and has also been found in different human tissues such as lung, skin, cervix, ciliary body and retinal pigment epithelium (50,51). Major protective effect of lycopene is its antioxidant effect through inactivation of ROS and quenching of free radicals (52). As a potent antioxidant, it has been studied as a potential protective agent in NAFLD. Studies investigating lycopene in NAFLD are listed on Table 1.
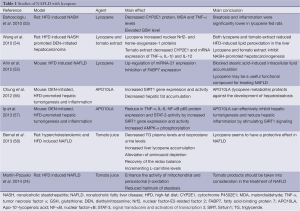
Full table
A study examining the preventive role of lycopene in NASH was conducted on rats fed a high-fat diet (HFD) (53). Supplementation with lycopene lowered serum malondialdehyde (MDA) and TNF-α levels and elevated liver GSH level (P<0.001). Additionally, a decreased CYP2E1 protein with increasing lycopene dose was observed in rats fed a HFD. Steatosis and inflammation were significantly lower in lycopene-fed rats. They found that lycopene administration had a preventive effect on experimental NASH induced by HFD in the rat model and lycopene could reduce HFD-induced oxidative stress.
Several studies reported that intake of tomato juice appeared to have a protective effect on NAFLD in rats that are hypercholesterolemic and fed a HFD (24,58). Rats fed tomato juice supplemented diet had lower TG level in plasma and isoprostane content in urine indicating alleviation of oxidative stress. They also had enhanced activity of enzymes involved in mitochondrial and peroxisomal β-oxidation of long-chain fatty acids in NAFLD. Lycopene accumulation in the liver was observed in tomato juice supplemented rats. Others have reported significantly improved absorption of carotenoids linked to the intake of dietary fat (59).
Wang et al. (54) investigated the protective effect of lycopene and tomato extract supplementation on NASH-promoted hepatocarcinogenesis in rats. HFD-induced NASH-promoted diethylnitrosamine (DEN)-initiated hepatocarcinoma rat model was used in the study. Lycopene and tomato extract could inhibit NASH-promoted hepatocarcinogenesis through reduced oxidative stress but with different mechanisms. They observed significantly decreased cytochrome P450 2E1, inflammatory foci and mRNA expression of proinflammatory cytokines (TNF-α, IL-1β and IL-12) in the tomato extract fed group, but increased nuclear NF-E2-related factor-2 and heme oxygenase-1 proteins in the lycopene fed group.
Carotene-15,15'-monooxygenase (CMO-I) and carotene-9',10'-monooxygenase (CMO-II) are primary mammalian carotenoid cleavage enzymes. CMO-I cleaves β-carotene to two molecules of retinal, whereas CMO-II preferentially cleaves non-provitamin A carotenoids such as lycopene but also has affinity to other carotenoids such as β-carotene (60-63). CMO-I has been identified as a cytoplasmic enzyme, whereas CMO-II has been identified as a mitochondrial enzyme. These enzymes convert the carotenoids to biologically active metabolites (60). Lycopene may also interfere with the β-carotene and retinoid metabolism. It has been found that lycopene supplementation decreased the expression of CMO-I and PPAR-γ in the kidney and adrenal tissues of rats (64). Apo-10’-lycopenoic acid (APO10LA) a cleavage metabolite of lycopene, generated by CMO-II and had significant biological activities (56,62,65).
APO10LA supplementation significantly reduced DEN-initiated, HFD–promoted hepatic tumorigenesis and inflammation in C57BL/6J mice (57). Hepatic pro-inflammatory biomarkers including TNF-α, IL-6, NF-κB p65 protein expression, caspase-1 cleavage and activation of the oncogenic transcription factor STAT3 were significantly reduced in the liver tissue by APO10LA supplementation. These effects of APO10LA were associated with increased hepatic Sirtuin1 (SIRT1), protein and deacetylation of SIRT1 targets (NF-κB p65 and FoxO1) and AMP-activated protein kinase (AMPK) phosphorylation. Another study suggested significantly decreased hepatosteatosis together with increased SIRT1 gene expression and activity by APO10LA supplementation in the ob/ob mice with HFD induced hepatosteatosis model (56). SIRT1, an NAD+-dependent protein deacetylase, is expressed in various tissues including the liver (66) and plays an important role in the regulation of lipid metabolism (67) and deacetylation of many proteins such as p53, NF-κB, FOXO, liver X receptor (LXR) (68). Protective effect of overexpression of SIRT1 was found in HFD-induced fatty liver disease (69).
Ahn et al. (55) reported that HFD induced downregulation of miRNA-21 expression was reversed by lycopene. As a post-transcriptional regulator of gene expression, up-regulating miRNA-21 was achieved by lycopene through targeting the fatty acid-binding protein 7 (FABP7). FABPs are most active proteins in long chain fatty acid uptake and metabolism in the hepatocytes. They found that lycopene up-regulated the miRNA-21 and inhibited FABP7 expression and blocked stearic acid (SA) induced intracellular lipid accumulation. They also observed that miRNA could have an important role in hepatic function and its expression was changed by HFD in liver tissues in an animal model (55,70). Ahn et al. also reported that miR-21 expression was decreased in HFD-induced NASH and stearic acid treated Hepa 1-6 cells. NASH was associated with downregulation of miRNA-21 and upregulation of FABP7. FABPs are most active proteins in long chain fatty acid uptake and metabolism in the hepatocytes (71). FABP7 was one of the targets of miRNA-21, and it was directly and inversely associated with miR-21 (55). Lycopene normalized the effects of HFD and regulated the hepatic lipid metabolism in this model. It downregulated PPARγ and fatty acid synthase (FASN) and upregulated CPT1-α, LCAD, PPAR-α and Apoa4 in HFD induced NASH mouse model (55).
β-carotene and NAFLD
β-carotene is the most widely distributed carotenoid in yellow-orange and dark green fruits and vegetables (72). β-carotene is also the most abundant carotenoid in the liver (42). Among provitamin A carotenoids, β-carotene has the highest provitamin A activity because it is partly converted to vitamin A (73). β-carotene has a strong antioxidant effect through scavenging free radicals and physically quenching singlet oxygen (74). Major source of β-carotene in human diet is primarily green leafy vegetables, carrots, apricots, sweet potatoes, red palm oil, mature squashes, pumpkins, and mangoes (73,75,76). As a potent antioxidant, β-carotene has been studied as a potential protective agent in NAFLD. The studies reporting the effects of β-carotene and other carotenoids on NAFLD are summarized in Table 2.
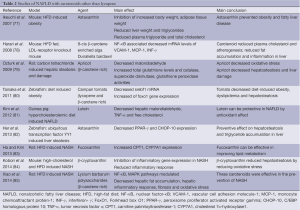
Full table
In vivo and in vitro experimental studies have shown potential preventive and therapeutic effects of β-carotene on hepatic inflammation, fibrosis (86) and cirrhosis (87). Another study reported that β-carotene could decrease hepatitis C virus induced-hepatosteatosis via inhibition of HCV RNA replication (88). Dietary β-carotene supplementation has been found to have a protective effect on liver damage. In rats with monocrotaline-induced steatosis, fat accumulation and hemorrhages decreased in the liver with β-carotene supplementation (89).
9-cis β-carotene is an isomer of β-carotene. Harari et al. (78) showed that 9-cis β-carotene supplementation reduced plasma cholesterol concentrations and atherogenesis, and inhibited fat accumulation and inflammation in the livers of mice fed a HFD. This could be due to reduced mRNA levels of inflammatory genes such as vascular cell adhesion molecule-1 (VCAM-1), IL-1α, monocyte chemoattractant protein-1 (MCP-1), interferon-γ (INF-γ).
Hepatic protective effects of some β-carotene rich products have been shown in experimental studies. Administration of an herbal derivative, Lycium barbarum polysaccharides has been shown to have ameliorative effects on hepatic fibrosis, oxidative stress and inflammatory response in HFD induced NASH and cellular steatosis rat model (85).
A study conducted by Ozturk et al. (79) demonstrated that dietary intake of apricot could reduce the risk of hepatic steatosis and damage caused by free radicals. Apricot is a fruit that has a high content of carotenoids, largely β-carotene. Markers of oxidative stress MDA, total GSH levels, catalase, superoxide dismutase and GSH peroxidase activities were significantly altered in carbon tetrachloride induced hepatic steatosis and damage in Wistar rats. Oxidative stress was decreased and hepatic steatosis and damage were ameliorated in rats by β-carotene rich apricot feeding.
In another study, Campari tomato, which contains more β-carotene and lycopene than regular tomato, ameliorates diet-induced obesity, dyslipidemia and hepatosteatosis via downregulation of gene expression related to lipogenesis in the zebra fish model. Campari tomato decreased sterol regulatory element-binding transcription factor 1 (srebf1) mRNA by increase of forkhead box O1 (foxo1) gene expression, which may depend on high contents of β-carotene in this tomato strain (80).
In a human study, researchers found that NAFLD had inverse relationship with vitamin A nutritional status in individuals with class III obesity (90). Retinol and β-carotene serum levels were evaluated as a biochemical indicator. The researchers observed low retinol and β-carotene serum levels in the presence of the NAFLD. They also reported significant association between insulin resistance with retinol and β-carotene levels.
Other carotenoids and NAFLD
Other carotenoids such as astaxanthin, lutein, β-cryptoxanthin, and fucoxanthin have also shown a protective effect in NAFLD.
Hypolipidemic and antioxidant effects of astaxanthin supplementation have been observed in human clinical trials (91,92). Astaxanthin treatment prevented triglyceride accumulation and liver steatosis by inhibiting PPAR-γ in ubiquitous transcription factor YY1 induced zebrafish liver steatosis (82). In another study, astaxanthin prevented the development of hepatic steatosis and lowered plasma total cholesterol and triglyceride in obese mice fed a HFD (77).
A study was performed with lutein administration in hypercholesterolemic diet fed guinea pigs, which showed that hepatic free cholesterol, hepatic MDA and TNF-α were decreased in the lutein supplemented group. The lutein group also had lower NF-κB DNA binding activity. These antioxidant effects of lutein could be protective in NAFLD (81).
An experimental study evaluated the effect of β-cryptoxanthin in mice with high-cholesterol and HFD induced NASH. Comprehensive gene expression analysis was performed in the livers of the mice. β-cryptoxanthin reduced steatosis through alteration in the expression of genes associated with cell death, inflammatory responses, infiltration and activation of macrophages and other leukocytes, quantity of T cells, and free radical scavenging (84).
Supplementation with fucoxanthin reduced body weight, body and liver fat content, and improved liver function tests in obese premenopausal women with NAFLD (93). Consumption of fucoxanthin was effective in improving lipid and cholesterol metabolism by increasing CPT1, cholesterol 7α-hydroxylase1 (CYP7A1) in rats with a HFD. These results suggest that fucoxanthin could be helpful in preventing NAFLD (83).
Conclusions
NAFLD has become one of the most important chronic liver diseases in the developed countries with consistently increased prevalence. Its association with obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and progression to cirrhosis and HCC increases its clinical importance. Pathogenesis of the NAFLD is a very complex process and may have many mechanisms. Understanding the pathogenetic mechanisms may assist in developing new preventive and therapeutic strategies. Oxidative stress and proinflammatory processes play an important role in the pathogenesis. Carotenoids, which are antioxidant natural compounds, appear to have beneficial effects in the prevention and treatment of NAFLD. Antioxidant and anti-inflammatory properties are the leading mechanisms of actions of carotenoids. These effects modulate intracellular signaling pathways influencing gene expression and protein translation. Recent studies by Ip et al. have provided additional potential mechanisms of lycopene and its metabolites (94). They found that lycopene supplementation in beta-carotene-9',10'-oxygenase (BCO2)-knockout mice suppressed oncogenic signals, including Met mRNA, β-catenin protein, and mTOR complex 1 activation, which was associated with increased hepatic microRNA (miR)-199a/b and miR214 levels. These results provide novel experimental evidence that dietary lycopene can prevent HFD-promoted HCC incidence and multiplicity in mice, and may elicit different mechanisms depending on BCO2 expression. Future investigations are warranted to understand the precise mechanisms as well as potential preventive and therapeutic effects of carotenoids in NAFLD and HCC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Khachik F, Beecher GR, Smith JC Jr. Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem Suppl 1995;22:236-46. [PubMed]
- Agarwal S, Rao AV. Carotenoids and chronic diseases. Drug Metabol Drug Interact 2000;17:189-210. [PubMed]
- Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 2014;34:907-29. [PubMed]
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 2007;55:207-16. [PubMed]
- Johnson EJ. The role of carotenoids in human health. Nutr Clin Care 2002;5:56-65. [PubMed]
- Pan MH, Lai CS, Tsai ML, et al. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol Nutr Food Res 2014;58:147-71. [PubMed]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221-31. [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. [PubMed]
- Vanni E, Bugianesi E, Kotronen A, et al. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis 2010;42:320-30. [PubMed]
- Whalley S, Puvanachandra P, Desai A, et al. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med 2007;7:119-24. [PubMed]
- Suzuki A, Angulo P, Lymp J, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology 2005;41:64-71. [PubMed]
- Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of non-alcoholic fatty liver disease. Ann Intern Med 2005;143:722-8. [PubMed]
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124-31. [PubMed]
- Hassan K, Bhalla V, Ezz El Regal M, et al. Nonalcoholic fatty liver disease: A comprehensive review of a growing epidemic. World J Gastroenterol 2014;20:12082-101. [PubMed]
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011;9:524-530. [PubMed]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836-46. [PubMed]
- Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582-8. [PubMed]
- van der Poorten D, Milner KL, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449-57. [PubMed]
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11-8. [PubMed]
- Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191-8. [PubMed]
- Fabbrini E, Mohammed BS, Magkos F, et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008;134:424-31. [PubMed]
- Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343-51. [PubMed]
- Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003;46:3-19. [PubMed]
- Martín-Pozuelo G, Navarro-González I, González-Barrio R, et al. The effect of tomato juice supplementation on biomarkers and gene expression related to lipid metabolism in rats with induced hepatic steatosis. Eur J Nutr 2014. [Epub ahead of print]. [PubMed]
- Adiels M, Westerbacka J, Soro-Paavonen A, et al. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia 2007;50:2356-65. [PubMed]
- Seren S, Mutchnick M, Hutchinson D, et al. Potential role of lycopene in the treatment of hepatitis C and prevention of hepatocellular carcinoma. Nutr Cancer 2008;60:729-35. [PubMed]
- Rao AV, Ray MR, Rao LG. Lycopene. Adv Food Nutr Res 2006;51:99-164. [PubMed]
- Chew BP, Park JS. Carotenoid action on the immune response. J Nutr 2004;134:257S-61S. [PubMed]
- Aubert J, Begriche K, Knockaert L, et al. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol 2011;35:630-7. [PubMed]
- Schattenberg JM, Wang Y, Singh R, et al. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem 2005;280:9887-94. [PubMed]
- Ekström G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1). Biochem Pharmacol 1989;38:1313-9. [PubMed]
- Madan K, Bhardwaj P, Thareja S, et al. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin Gastroenterol 2006;40:930-5. [PubMed]
- Perlemuter G, Davit-Spraul A, Cosson C, et al. Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int 2005;25:946-53. [PubMed]
- Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr 2001;21:193-230. [PubMed]
- Rogue A, Renaud MP, Claude N, et al. Comparative gene expression profiles induced by PPARγ and PPARα/γ agonists in rat hepatocytes. Toxicol Appl Pharmacol 2011;254:18-31. [PubMed]
- Costet P, Legendre C, Moré J, et al. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem 1998;273:29577-85. [PubMed]
- Chao L, Marcus-Samuels B, Mason MM, et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest 2000;106:1221-8. [PubMed]
- Haukeland JW, Damås JK, Konopski Z, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006;44:1167-74. [PubMed]
- Hui JM, Hodge A, Farrell GC, et al. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004;40:46-54. [PubMed]
- Wieckowska A, Papouchado BG, Li Z, et al. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008;103:1372-9. [PubMed]
- Schwabe RF, Uchinami H, Qian T, et al. Differential requirement for c-Jun NH2-terminal kinase in TNFalpha- and Fas-mediated apoptosis in hepatocytes. FASEB J 2004;18:720-2. [PubMed]
- Vitaglione P, Morisco F, Caporaso N, et al. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr 2004;44:575-86. [PubMed]
- Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 1993;328:1828-35. [PubMed]
- Lee KS, Buck M, Houglum K, et al. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 1995;96:2461-8. [PubMed]
- Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res 2012;347:245-56. [PubMed]
- Kim MS, Kung S, Grewal T, et al. Methodologies for investigating natural medicines for the treatment of nonalcoholic fatty liver disease (NAFLD). Curr Pharm Biotechnol 2012;13:278-91. [PubMed]
- Park SK, Lee HJ, Lee DH, et al. Associations of non alcoholic fatty liver with the metabolic syndrome and serum carotenoids. J Prev Med Public Health 2008;41:39-44. [PubMed]
- Erhardt A, Stahl W, Sies H, et al. Plasma levels of vitamin E and carotenoids are decreased in patients with Nonalcoholic Steatohepatitis (NASH). Eur J Med Res 2011;16:76-8. [PubMed]
- van Breemen RB, Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett 2008;269:339-51. [PubMed]
- Khachik F, Carvalho L, Bernstein PS, et al. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med (Maywood) 2002;227:845-51. [PubMed]
- Rao AV, Agarwal S. Role of Antioxidant Lycopene in Cancer and Heart Disease. Journal of the American College of Nutrition 2000;19:563-9. [PubMed]
- Britton G. Carotenoids 1: structure and properties of carotenoids in relation to function. FASEB J 1995;9:1551-8. [PubMed]
- Bahcecioglu IH, Kuzu N, Metin K, et al. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet Med Int 2010;2010.pii:262179.
- Wang Y, Ausman LM, Greenberg AS, et al. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer 2010;126:1788-96. [PubMed]
- Ahn J, Lee H, Jung CH, et al. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol Nutr Food Res 2012;56:1665-74. [PubMed]
- Chung J, Koo K, Lian F, et al. Apo-10'-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr 2012;142:405-10. [PubMed]
- Ip BC, Hu KQ, Liu C, et al. Lycopene metabolite, apo-10'-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res (Phila) 2013;6:1304-16. [PubMed]
- Bernal C, Martín-Pozuelo G, Lozano AB, et al. Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J Nutr Biochem 2013;24:1870-81. [PubMed]
- Unlu NZ, Bohn T, Clinton SK, et al. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr 2005;135:431-6. [PubMed]
- Ford NA, Elsen AC, Erdman JW Jr. Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr Res 2013;33:733-42. [PubMed]
- Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15'-monooxygenase. J Biol Chem 2002;277:23942-8. [PubMed]
- Hu KQ, Liu C, Ernst H, et al. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 2006;281:19327-38. [PubMed]
- Kiefer C, Hessel S, Lampert JM, et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001;276:14110-6. [PubMed]
- Zaripheh S, Nara TY, Nakamura MT, et al. Dietary lycopene downregulates carotenoid 15,15'-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr 2006;136:932-8. [PubMed]
- Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr 2012;96:1214S-22S. [PubMed]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 2012;13:225-38. [PubMed]
- Lomb DJ, Laurent G, Haigis MC. Sirtuins regulate key aspects of lipid metabolism. Biochim Biophys Acta 2010;1804:1652-7.
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 2010;5:253-95. [PubMed]
- Pfluger PT, Herranz D, Velasco-Miguel S, et al. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 2008;105:9793-8. [PubMed]
- Hand NJ, Master ZR, Le Lay J, et al. Hepatic function is preserved in the absence of mature microRNAs. Hepatology 2009;49:618-26. [PubMed]
- Haunerland NH, Spener F. Fatty acid-binding proteins--insights from genetic manipulations. Prog Lipid Res 2004;43:328-49. [PubMed]
- Rodriguez-Amaya DB, Kimura M, Godoy HT, et al. Updated Brazilian database on food carotenoids: Factors affecting carotenoid composition. J Food Compost Anal 2008;21:445-63.
- Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005;26:459-516. [PubMed]
- Diplock AT. Antioxidant nutrients and disease prevention: an overview. Am J Clin Nutr 1991;53:189S-93S. [PubMed]
- Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev 2008;37:2558-74. [PubMed]
- Maiani G, Castón MJ, Catasta G, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res 2009;53:S194-218. [PubMed]
- Ikeuchi M, Koyama T, Takahashi J, et al. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci Biotechnol Biochem 2007;71:893-9. [PubMed]
- Harari A, Harats D, Marko D, et al. A 9-cis beta-carotene-enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. J Nutr 2008;138:1923-30. [PubMed]
- Ozturk F, Gul M, Ates B, et al. Protective effect of apricot (Prunus armeniaca L.) on hepatic steatosis and damage induced by carbon tetrachloride in Wistar rats. Br J Nutr 2009;102:1767-75. [PubMed]
- Tainaka T, Shimada Y, Kuroyanagi J, et al. Transcriptome analysis of anti-fatty liver action by Campari tomato using a zebrafish diet-induced obesity model. Nutr Metab (Lond) 2011;8:88. [PubMed]
- Kim JE, Clark RM, Park Y, et al. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr Res Pract 2012;6:113-9. [PubMed]
- Her GM, Pai WY, Lai CY, et al. Ubiquitous transcription factor YY1 promotes zebrafish liver steatosis and lipotoxicity by inhibiting CHOP-10 expression. Biochim Biophys Acta 2013;1831:1037-51.
- Ha AW, Kim WK. The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet. Nutr Res Pract 2013;7:287-93. [PubMed]
- Kobori M, Ni Y, Takahashi Y, et al. β-Cryptoxanthin alleviates diet-induced nonalcoholic steatohepatitis by suppressing inflammatory gene expression in mice. PLoS One 2014;9:e98294. [PubMed]
- Xiao J, Xing F, Huo J, et al. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci Rep 2014;4:5587. [PubMed]
- Seifert WF, Bosma A, Hendriks HF, et al. Beta-carotene (provitamin A) decreases the severity of CCl4-induced hepatic inflammation and fibrosis in rats. Liver 1995;15:1-8. [PubMed]
- Wardi J, Reifen R, Aeed H, et al. Beta-carotene attenuates experimentally induced liver cirrhosis in rats. Isr Med Assoc J 2001;3:151-4. [PubMed]
- Liu Q, Bengmark S, Qu S. Nutrigenomics therapy of hepatisis C virusn induced-hepatosteatosis. BMC Gastroenterol 2010;10:49. [PubMed]
- Baybutt RC, Molteni A. Dietary beta-carotene protects lung and liver parenchyma of rats treated with monocrotaline. Toxicology 1999;137:69-80. [PubMed]
- Villaça Chaves G, Pereira SE, Saboya CJ, et al. Non-alcoholic fatty liver disease and its relationship with the nutritional status of vitamin A in individuals with class III obesity. Obes Surg 2008;18:378-85. [PubMed]
- Iwamoto T, Hosoda K, Hirano R, et al. Inhibition of low- density lipoprotein oxidation by astaxanthin. J Atheroscler Thromb 2000;7:216-22. [PubMed]
- Yoshida H, Yanai H, Ito K, et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010;209:520-3. [PubMed]
- Abidov M, Ramazanov Z, Seifulla R, et al. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metab 2010;12:72-81. [PubMed]
- Ip BC, Liu C, Ausman LM, et al. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev Res (Phila) 2014;7:1219-27. [PubMed]


