Hepatoid carcinoma of the pancreas combined with serous cystadenoma: a case report and review of the literature
Introduction
Hepatoid carcinoma (HC) is a neoplasm exhibiting features of hepatocellular carcinoma (HCC) in terms of morphology and immunohistochemistry which grows outside the liver. Since its first description by Ishikura et al. in the stomach which is the most common location, it has been described in different organs such as lung, pancreas, esophagus, papilla of Vater, colon, urinary bladder, renal pelvis, ovaries, biliary tract and the gallbladder (1-9). The true incidence and exact behavior of pancreatic HC still remain unclear as only few case reports are available in the literature. This paper reports a case of incidentally detected HC combined with a serous cystadenoma arising from pancreas in a 47-year-old male patient.
Case report
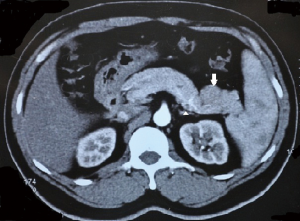
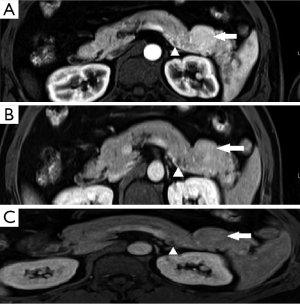
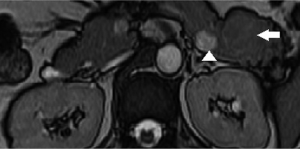
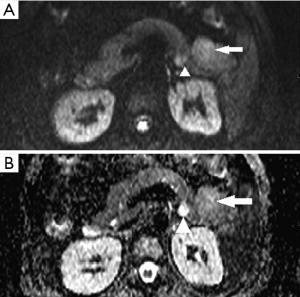
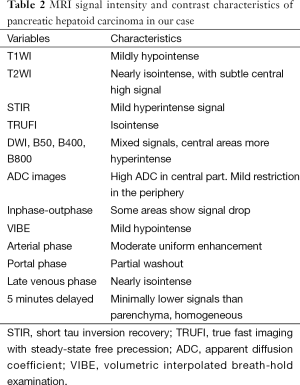
A 47-year-old gentleman was referred to our institute because of an incidentally detected pancreatic mass while being evaluated for renal calculi. He had recurrent left sided loin to groin pain and associated backache for 6 months. Physical examinations on admission were unremarkable. Ultrasound of abdomen showed bilateral renal calculi and a fairly well defined hypoechoic lesion measuring 2.3 cm × 2 cm in relation to the tail of pancreas with mild internal vascularity. Contrast enhanced computed tomography (CECT) of abdomen showed two morphologically different lesions in the distal body and proximal tail of pancreas (Figure 1). Larger lesion was with well-defined margins and partially exophytic from the tail with mild heterogeneous enhancement. Smaller lesion was moderately enhancing and situated in the junction of body and tail of pancreas close to posterior margin. The margins of lesion demonstrated higher enhancement. MRI also revealed similar findings and a small calculus in the neck of gall bladder. The distal lesion was of size 3.13 cm × 2.98 cm × 2.67 cm and mildly hypointense in T1W1, nearly isointense in T2W1 image. It showed moderate uniform enhancement in arterial phase, partial washout in portal phase and further wash out in hepatic venous phase (Figure 2). The tumor was mildly hypointense in VIBE (volumetric interpolated breath-hold examination) sequence, isointense in TRUFI (true fast imaging with steady-state free precession) sequence and minimally hyperintense in diffusion weighted imaging (DWI)-B400 with high apparent diffusion coefficient (ADC) in the central part with peripheral areas showing mild restriction in ADC images (Figures 3,4). The proximal lesion was of size 1.55 cm × 2.11 cm × 1.57 cm with moderate enhancement at periphery and a central non enhancing area (Figure 2). Laboratory panel showed (our lab’s normal range in brackets); CA 19-9: 13.61 U/mL (0-35 U/mL), carcinoembryonic antigen (CEA): 1.68 ng/mL (0-3 ng/mL), chromogranin A: 41.35 ng/mL (<100 ng/mL), amylase: 113 U/L(28-100 U/L) and lipase: 153 U/L (13-60 U/L). With a possibility of non-functional neuroendocrine tumor, patient was taken up for surgery. Laparoscopic distal pancreatectomy with splenectomy and cholecystectomy were done. There was no evidence of any hepatic, peritoneal or lymph node metastasis.
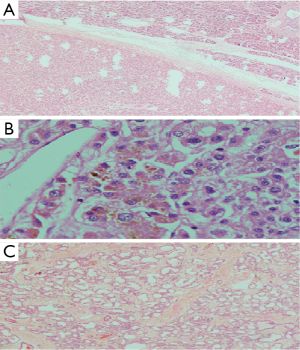
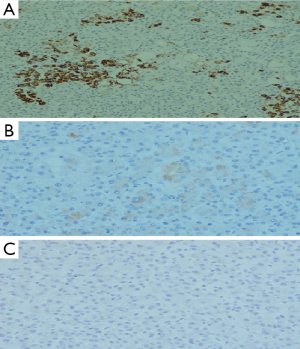
Cut section of the distal lesion was well circumscribed, encapsulated and let out bile. Cut section of the proximal tumor showed tiny cystic spaces. Microscopic examination of the distal tumor revealed an encapsulated neoplasm with features of hepatocellular tissue. It was composed of cells in trabecular pattern separated by sinusoids (Figure 5A). Tumor cells had moderate eosinophilic granular cytoplasm and vescicular nucleus with prominent nucleolus and intranuclear eosinophilic inclusions. Canalicular and intercellular bile plugs were seen (Figure 5B). There was no bile ductule or portal triad. Kupffer cells were, however, present. Smaller proximal neoplasm had multiple cystically dilated spaces lined by cuboidal cells (Figure 5C). Peripancreatic lymph nodes showed reactive hyperplasia only. Resection margins were negative for neoplasm. Immunohistochemically the distal tumor was strongly positive for hepatocyte specific antigen (Figure 6A), glypican 3 (focal) and cytokeratins (AE1/AE3, CK8 and CK18), with weak positivity for alphafetoprotein (Figure 6B). Tumor cells were immunonegative for chromogranin (Figure 6C), EMA and CK-7. The liver like tissue showed CD 34 expressing capillarized sinusoids. Gallbladder revealed no specific pathology. Based on these findings the distal tumor was diagnosed as hepatoid tumor of pancreas and the proximal one as serous microcystic cystadenoma. Alpha fetoprotein (AFP) detected 2 weeks post resection was 1.75 IU/mL (<6.0 IU/mL). At eighth month of follow up AFP is 3.14 IU/mL and CECT of abdomen shows no evidence of recurrence.
Discussion
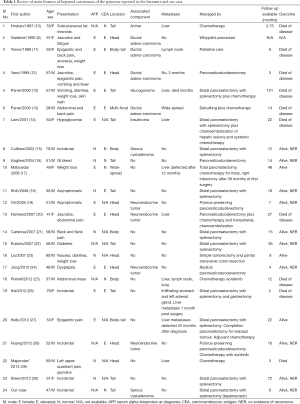
HC of the pancreas is an extremely rare neoplasm and only 23 cases have been reported in the literature so far (Table 1). The first pancreatic form of HC was reported by Hruban et al. in 1987 (10). The etiology and pathogenesis of pancreatic HC is still not clear and proposed theories of its origin mainly revolve around the transdifferentiation of pancreatic cells into hepatocytes and the common embryologic foregut derivation of the pancreas and liver (13,23). Though reported ages at presentation range from 21 to 80, most patients (16/23, 69%) were above 50 years of age. There is a clear male predominance (14/23, 60%). The most common presenting symptom was pain (6/23, 26%), either back or epigastric, followed by jaundice and weight loss. Many cases reported in the literature were asymptomatic or incidentally discovered (6/23, 26%) like our patient. More than 50% of Pancreatic HCs were located in the body or tail of the pancreas (13/23). Liver and lymph nodes are the most frequent sites of metastasis (23,31).
Full table
Preoperative diagnosis is often challenging, even with appropriate imaging and cytological examination. In a contrast enhanced CT scan of abdomen HC often reveals as a heterogeneous mass with irregular enhancement (32) as it is observed in this reported case. MR imaging features of pancreatic HC in our case are similar to neuroendocrine tumors, except for mild diffusion signals in the lesion (Table 2). When diagnosing primary pancreatic HC, it is important to exclude metastatic HCC by clinical and pathological examination. Serum AFP levels is found to be elevated in most cases and can be used postoperatively to assess completeness of resection and extent of response to chemotherapy, and to detect recurrence of the tumor during follow up (24,31,33,34). However, AFP secretion can also be noticed in other pancreatic tumors like acinar and ductal neoplasms, neuroendocrine tumors and pancreatoblastomas (13,35,36). Diagnosis mainly depends on specific pathological findings. The characteristic pathological features are medium to large polygonal cells with eosinophilic to clear cytoplasm, vesicular nuclei and prominent nucleoli growing in a perisinusoidal pattern, along with the demonstration of the presence of bile and an immunohistochemical profile characteristic of HCC (37). Bile production in the tumor as in our case though a rare finding is more conclusive for hepatoid neoplasm (13). IHC markers used for diagnosis include immunoreactivity with polyclonal antibodies against AFP, CEA, and more specific markers like hepatocyte-specific Hep-Par1 antibody and albumin mRNA detected by in situ hybridization (13,20).
Full table
Immunohistochemical profiling with cytokeratins (CK) can be helpful in differentiating hepatoid tumours from HCC. Hepatoid tumors are most often positive for pancytokeratin marker AE1/AE3 (92%), CK 19 (94-100%) and CK18, and negative for CK7 (38,39). CK20 positivity is seen in about 25-47% of hepatoid neoplasms (38,39). CK19 and 20 expression is very rarely seen in HCC (38-40). Monoclonal antibody HepPar1 is expressed by normal and neoplastic hepatocytes, and is considered more sensitive than AFP to diagnose HCC. It is also found to be positive in some cases of HCs, but diffuse positivity for HepPar1 is more consistent with HCC than hepatoid neoplasm (20,38,39). HCs can be differentiated from other AFP producing tumors like pancreatoblastoma and acinar cell carcinoma of pancreas by their histopathological features and by expression of liver specific proteins on immunohistochemistry. The presence of small acinar structures, mesenchymal components, or the characteristic squamous corpuscles would favor a diagnosis of pancreatoblastoma and in case of acinar cell carcinoma immunoreactivity with trypsin, chymotrypsin, or lipase can be detected.
Pancreatic HC can present in pure forms or in association with histologically different components such as adenocarcinoma or neuroendocrine tumors (20,24). Those with pure hepatoid or hepatocellular differentiation seem to have better survival and recurrence rates than patients with mixed-type tumors (27). In our case it was associated with a serous microcystic cystadenoma in an adjacent location. Only one out of 23 cases reported in the literature had microcystic cystadenoma as an associated component (15) (Table 1).
Surgical resection is considered as the mainstay of treatment whenever possible. Some authors have advocated adjuvant chemotherapy because of the metastatic potential of the tumor while others have found no added benefit (20). A certain degree of response to chemotherapy and multi-target tyrosine kinase inhibitor sorafenib was reported in locally unresectable, metastatic or recurrent disease (25). Petrelli et al. reported a case of metastatic HC (mainly to the liver, lymph nodes, and lungs) in a 37-year-old male treated with the multi-target tyrosine kinase inhibitor sorafenib (400 mg BD). It provided disease control for 8 months, which was confirmed by imaging and biochemical data. But they were forced to discontinue the therapy because of worsening liver failure (25). In 2012, Karayiannakis et al. reported an overall survival of 20 months in a 60-year-old female patient with hepatoid adenocarcinoma of the gallbladder treated with surgery followed by sorafenib for 15 months until her disease progressed (33). Lucas et al. reported a case of hepatoid adenocarcinoma of peritoneal cavity (which was closely related to colon) treated with FOLFOX (5-fluorouracil, leucovorin and oxaliplatin) after complete resection of the tumor. They report more than 3 years of follow up without any evidence of recurrence (38). Successful disease control with a regimen of 5-FU plus paclitaxel has been reported in a 64-year-old male patient with metastatic gastric hepatoid adenocarcinoma by Takeyama et al. in 2007 (34).
HCs of the gastrointestinal tract are generally considered as very aggressive neoplasms and have an unfavorable prognosis (39). Survival appears to be mainly depends upon the extent of the disease and completeness of resection. Longest survival reported with metastatic HC after resection is 8.5 years (13) (Table 1). Steen et al. reported more than 5-year survival without any recurrence after complete resection of a HC localized to the tail of pancreas, without any adjuvant therapy (30). Owing to its rarity further studies and long term follow up are needed to standardize the treatment and to correctly assess prognostic features.
Conclusions
In conclusion, though HC of pancreas is extremely rare it should be considered in preoperative differential diagnosis of pancreatic tumors especially when the lesion is associated with atypical clinical presentation and image findings. The diagnosis is mainly based on histopathological and immunohistochemical features and an early detection is vital as complete resection of the tumor appears to be the best option of the treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Ishikura H, Fukasawa Y, Ogasawara K, et al. An AFP-producing gastric carcinoma with feature of hepatic differentiation: a case report. Cancer 1985;56:840-8. [PubMed]
- Ishikura H, Kanda M, Ito M, et al. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol 1990;417:73-80. [PubMed]
- Tochigi N, Kishimoto T, Supriatna Y, et al. Hepatoid carcinoma of the ovary: a report of three cases admixed with a common surface epithelial carcinoma. Int J Gynecol Pathol 2003;22:266-71. [PubMed]
- Tanigawa H, Kida Y, Kuwao S, et al. Hepatoid adenocarcinoma in Barrett’s esophagus associated with achalasia: first case report. Pathol Int 2002;52:141-6. [PubMed]
- Lopez-Beltran A, Luque RJ, Quintero A, et al. Hepatoid adenocarcinoma of the urinary bladder. Virchows Arch 2003;442:381-7. [PubMed]
- Gardiner GW, Lajoie G, Keith R. Hepatoid adenocarcinoma of the papilla of Vater. Histopathology 1992;20:541-4. [PubMed]
- Liu Q, Bannan M, Melamed J, et al. Two cases of hepatoid adenocarcinoma of the intestine in association with inflammatory bowel disease. Histopathology 2007;51:123-5. [PubMed]
- van den Bos IC, Hussain S, Dwarkasing R, et al. Hepatoid adenocarcinoma of the gallbladder: a mimicker of hepatocellular carcinoma. Br J Radiol 2007;80:e317-20. [PubMed]
- Abdullah A, Jenkins-Mosure K, Lewis T, et al. Primary hepatoid carcinoma of the biliary tree: a radiologic mimicker of Klatskin-type tumor. Cancer Imaging 2010;10:198-201. [PubMed]
- Hruban RH, Molina JM, Reddy MN, et al. A neoplasm with pancreatic and hepatocellular differentiation presenting with subcutaneous fat necrosis. Am J Clin Pathol 1987;88:639-45. [PubMed]
- Tanno S, Obara T, Fujii T, et al. Alpha-fetoprotein-producing adenocarcinoma of the pancreas presenting focal hepatoid differentiation. Int J Pancreatol 1999;26:43-7. [PubMed]
- Yano T, Ishikura H, Wada T, et al. Hepatoid adenocarcinoma of the pancreas. Histopathology 1999;35:90-2. [PubMed]
- Paner GP, Thompson KS, Reyes CV. Hepatoid Carcinoma of the Pancreas. Cancer 2000;88:1582-9. [PubMed]
- Lam K, Lo C, Wat M, et al. Malignant insulinoma with hepatoid differentiation: a unique case with alpha-fetoprotein production. Endocr Pathol 2001;12:351-4. [PubMed]
- Cuilliere P, Lazure T, Bui M, et al. Solid adenoma with exclusive hepatocellular differentiation: a new variant among pancreatic benign neoplasms? Virchows Arch 2002;441:519-22. [PubMed]
- Hughes K, Kelty S, Martin R. Hepatoid carcinoma of the pancreas. Am Surg 2004;70:1030-3. [PubMed]
- Matsueda K, Yamamoto H, Yoshida Y, et al. Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist II (PIVKA-II) and -fetoprotein (AFP). J Gastroenterol 2006;41:1011-9. [PubMed]
- Shih NN, Tsung JS, Yang AH, et al. A unique pancreatic tumor with exclusive hepatocytic differentiation. Ann Clin Lab Sci 2006;36:216-21. [PubMed]
- Oh HJ, Cheung DY, Kim TH. A case of hepatoid carcinoma of the pancreas Korean J Gastroenterol 2006;47:389-93. (in Korean). [PubMed]
- Hameed O, Xu H, Saddeghi S, et al. Hepatoid carcinoma of the pancreas: a case report and literature review of a heterogeneous group of tumors. Am J Surg Pathol 2007;31:146-52. [PubMed]
- Cardona D, Grobmyer S, Crawford JM. Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch 2007;450:225-9. [PubMed]
- Kubota K, Kita J, Rokkaku K. Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol 2007;13:4270-3. [PubMed]
- Liu CZ, Hu SY, Wang L, et al. Hepatoid carcinoma of the pancreas: a case report. Chin Med J (Engl) 2007;120:1850-2. [PubMed]
- Jung JY, Kim YJ, Kim HM, et al. Hepatoid carcinoma of the pancreas combined with neuroendocrine carcinoma. Gut Liver 2010;4:98-102. [PubMed]
- Petrelli F, Ghilardi M, Colombo S, et al. A rare case of metastatic pancreatic hepatoid carcinoma treated with sorafenib. J Gastrointest Cancer 2012;43:97-102. [PubMed]
- Kai K, Nakamura J, Ide T, et al. Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: a case report and literature review. Pathol Int 2012;62:485-90. [PubMed]
- Kelly PJ, Spence R, Dasari BV, et al. Primary hepatocellular carcinoma of the pancreas: a case report and review of the heterogeneous group of pancreatic hepatoid carcinomas. Histopathology 2012;60:1012-5. [PubMed]
- Huang SC, Chang HC, Yeh TS, et al. Hepatoid microcarcinoma of the pancreas: a case report and review of the literature. Chang Gung Med J 2012;35:285-91. [PubMed]
- Majumder S, Dasanu CA. Hepatoid variant of pancreatic cancer: insights from a case and literature review. JOP 2013;14:442-5. [PubMed]
- Steen S, Wolin E, Geller SA, et al. Primary hepatocellular carcinoma (“hepatoid” carcinoma) of the pancreas: a case report and review of the literature. Clin Case Rep 2013;1:66-71. [PubMed]
- Xin BB, Li JA, Han X, et al. Successful treatment of a case with pancreatic neuroendocrine carcinoma with focal hepatoid differentiation: a case report and literature review. Int J Clin Exp Med 2014;7:3588-94. [PubMed]
- Kim KA, Park CM, Kim CH, et al. Hepatocellular carcinoma in an ectopic liver: CT findings. Eur Radiol 2003;13:L45-7. [PubMed]
- Karayiannakis AJ, Kakolyris S, Giatromanolaki A, et al. Hepatoid Adenocarcinoma of the Gallbladder: Case Report and Literature Review. J Gastrointest Cancer 2012;43 Suppl 1:139-44. [PubMed]
- Takeyama H, Sawai H, Wakasugi T, et al. Successful paclitaxel-based chemotherapy for an alpha-fetoprotein-producing gastric cancer patient with multiple liver metastases. World J Surg Oncol 2007;5:79. [PubMed]
- Chan MH, Shing MM, Poon TC, et al. Alpha-fetoprotein variants in a case of pancreatoblastoma. Ann Clin Biochem 2000;37:681-5. [PubMed]
- Zhu X, Yong H, Zhang L, et al. Pure alpha-fetoprotein-producing neuroendocrine carcinoma of the pancreas: a case report. BMC Gastroenterology 2015;15:16. [PubMed]
- Lowe CJ Jr, Riepe SP, Wood WC. Hepatocellular carcinoma presenting as a pancreatic head mass: report of an unusual case. Am J Clin Oncol 1997;20:509-10. [PubMed]
- Lucas ZD, Shah M, Trivedi A, et al. Hepatoid adenocarcinoma of the peritoneal cavity: Prolonged survival after debulking surgery and 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX) therapy. J Gastrointest Oncol 2012;3:139-42. [PubMed]
- Su JS, Chen YT, Wang RC, et al. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: A literature review. World J Gastroenterol 2013;19:321-7. [PubMed]
- Terracciano LM, Glatz K, Mhawech P, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol 2003;27:1302-12. [PubMed]


