Carcinosarcoma of the bile duct: a case report and review of literature
Introduction
Carcinosarcomas, also known as sarcomatoid carcinomas, are very rare tumors consisting of both epithelial and mesenchymal elements. These tumors have been reported in many different organs, including the pancreas, lung, uterus, ovary, esophagus, stomach and kidney (1-5). However, carcinosarcoma of the biliary tree is rare, with only a handful of cases reported in the bile ducts (6-11). We report an interesting case of a bile duct carcinosarcoma and a brief literature review.
Case presentation
A 91-year-old female patient was referred to the hepato-pancreato-biliary (HPB) service for further management of an intrabiliary mass near the biliary confluence detected on her CT scan (Figure 1). She had elevated liver enzymes [aspartate aminotransferase (AST): 133 U/L; alanine aminotransferase (ALT): 141 U/L and alkaline phosphate (ALP): 728 U/L], but bilirubin was within the normal range. Tumor markers were abnormal; alpha-fetoprotein (αFP), carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) were all elevated at 6.7 ng/mL, 5.8 ng/mL and 147 U/mL, respectively.
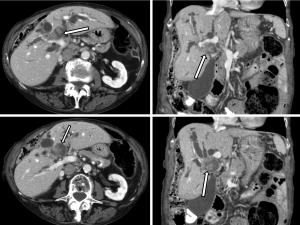
The consensus at a multi-disciplinary HPB tumor conference was an intraluminal soft tissue mass at the confluence of the left and right main bile ducts resulting in marked bilobar intrahepatic biliary dilatation, suspicious for a papillary hilar cholangiocarcinoma. A decision was made for limited surgical resection of the hilar cholangiocarcinoma, peri-hilar lymphadenectomy and a hepatojejunostomy without a liver resection due to patient’s age and personal preference to avoid a major hepatectomy. Additionally, given the difficulties of palliating jaundice in the setting of a large intraductal tumor, even a limited resection was considered preferable to non-operative decompression.
Intraoperatively, after exclusion of peritoneal metastases, a large papillary intrabiliary duct tumor arising from the biliary confluence was found and a complete gross resection was achieved by removing the biliary confluence and the tumor in-situ, and hepatojejunostomy was fashioned to the right, left and caudate ducts separately. The patient recovered uneventfully but the disease recurred after 2 months and she passed away soon after.
Histological features
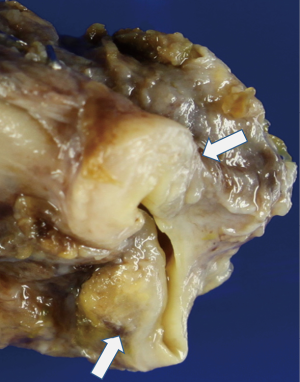
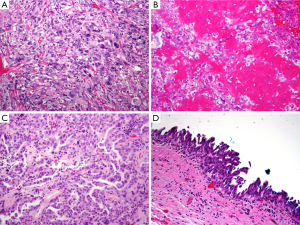
On gross examination, the tumor was primarily exophytic with masses seen within both the right and left hepatic ducts. An area of mural invasion was noted involving the right hepatic duct wall (Figure 2). Histologically, both the exophytic and murally invasive components were composed primarily of sarcomatoid elements, mostly in the form of spindle and pleomorphic tumor cells with prominent mitotic activity (Figure 3A). A minor component of the tumor showed osteogenic differentiation with formation of malignant osteoid (Figure 3B). The carcinoma component was minor, constituting about 10% of the entire tumor with morphological features of biliary adenocarcinoma and the overall architectural pattern being papillary (Figure 3C). Notably, the underlying bile duct epithelium showed foci of low and high grade dysplasia (Figure 3D).
Discussion
Carcinosarcomas are pathologically characterized by the presence of both epithelial and mesenchymal components within the same tumor. The most common site for carcinosarcoma of the biliary tract is the gallbladder with fewer than 100 cases reported in the literature; comparatively, there are only 7 cases of carcinosarcoma of the bile ducts reported in the English literature to date (Table 1) (6-13).
The pathogenesis of these rare tumors is uncertain but several hypotheses exist. It has been theorized that carcinosarcoma arises from totipotent stromal stem cells that are capable of divergent differentiation. Another postulation is the collision tumor theory that suggests the distinct and concurrent malignant proliferation of both the epithelial and mesenchymal components within the same tissue. It has also been suggested that a carcinoma can transform into a sarcoma by metaplastic transformation (14-16). A distinguishing feature of a true carcinosarcoma is the biphasic nature of the tumor with a lack of transition between the two epithelial and sarcomatous components as opposed to a poorly differentiated carcinoma with spindle cell pattern (6,14). The sarcomatous element commonly consists of undifferentiated spindle cells and a variety of heterogeneous components such as, chondro-, osteo-, leiomyo-, rhabdomyosarcoma cells (Table 1). The epithelial element usually consists of adenocarcinoma and occasionally, components such as squamous-, small cell- and undifferentiated carcinomas (17-20). Genetic analysis has been utilized to evaluate the pathogenesis of these tumors (21). They have also been sub-classified into two sub-groups: one with predominant sarcomatous differentiation or predominantly carcinomatous differentiation.
The demographics of biliary carcinosarcoma are similar to that of gallbladder adenocarcinoma, with the majority occurring in elderly women and strongly associated with cholelithiasis (22). The prognosis is dismal, largely extrapolated from the experience of gallbladder carcinosarcomas. The reported median survival time of patients with carcinosarcomas of the gallbladder after surgical resection was only 7 months with a 1-, 2-, and 3-year survival rates of 37.2%, 31.0%, and 31.0% respectively (23). The median time to recurrence for patients who died was less than 2 months (23). The TNM staging system is a valuable prognostic tool in gallbladder carcinoma but has little role in gallbladder carcinosarcoma; a recent review demonstrated that the survival time is in matter of months regardless of their tumor stage (24-29).
In general, carcinosarcomas are locally aggressive tumors with a propensity to metastasize systemically even in early stages (7,30). It been suggested that the aggressiveness of the tumor depends on the sarcomatoid component, as they metastasize to the lymph nodes and distant organs more readily. Interestingly, it is also the sarcomatoid component that forms the bulk of polypoidal element that leads to an earlier presentation as it obstructs the bile ducts, in contrast to gallbladder carcinosarcomas, where the carcinomatoid element forms the infiltrative component of the tumor (6,7,9,11,17). Kubota et al. (31) studied ki-67 in a case of gallbladder carcinosarcoma and compared it to a cohort (n=11) of patients with ordinary gallbladder adenocarcinoma: the ki-67 index in the spindle-cell component was higher than that in the epithelial component, which may account for the more aggressive biological behavior of the carcinosarcoma. This observation is consistent with other studies reporting that Ki-67 has prognostic value in various types of carcinosarcoma and other malignant tumors as well (31,32). Due to the rarity of bile duct carcinosarcoma, its true prognosis and clinicopathological features are not well established. Based on the reported cases, the survival time can vary widely, from 2 months to 5 years of disease-free survival (Table 1).
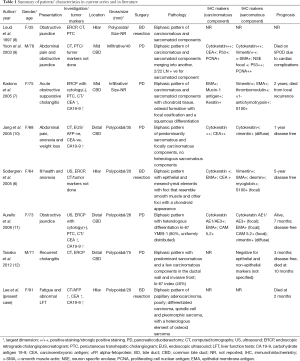
Full table
Management of all biliary malignancy, including carcinosarcoma, remains challenging, and this is particularly true for those arising from the proximal biliary tree. The strategy has largely been extrapolated from the experience in treating cholangiocarcinoma, with resection as the mainstay of treatment. As is also true for the more common biliary adenocarcinoma, there is no clear evidence that chemotherapy or radiotherapy confers any survival benefit, either as adjuvant treatment or in the palliative setting (20,25). However, judging from the poor results, it is clear that surgery alone is inadequate. Adjuvant chemotherapy has been used in carcinosarcomas of the female genital tract but with disappointing results and the role of radiation is currently unclear as well (6,7,20,30). Lumsden et al. reported improved survival with the use of intracavitary radiotherapy via a T-Tube for a case of a gallbladder carcinosarcoma (28).
This case report includes interesting radiologic and histologic features of carcinosarcoma of the bile duct. Carcinosarcomas often demonstrate unusual gross and radiographic features. Polypoid growth, which was also observed in this patient, was reported to be the characteristic gross feature of carcinosarcomas (5,7,8,33). The features of intraductal expansion, distension of the biliary confluence by the polypoidal tumor and subsequent proximal biliary tree obstruction were noted in our patient and are illustrated in Figure 1. This growth pattern is also seen in papillary cholangiocarcinoma, which is often a more indolent disease. As the present cases illustrates, the radiographic features of carcinosarcoma and papillary cholangiocarcinoma may be indistinguishable.
Conclusions
We report a case of carcinosarcoma of the bile duct with biphasic areas of papillary adenocarcinoma, poorly differentiated carcinoma intermingled with spindle cell and pleomorphic sarcoma and a heterologous element of osteoid sarcoma. To the best of our knowledge, this is the eighth case of biliary carcinosarcoma, it’s pathological and immunohistochemical characteristics define and distinguish it from a hilar cholangiocarcinoma. Complete resection is the optimal mode of treatment when possible, but the prognosis for most patients is poor; roles for chemotherapy and/or radiation therapy are undefined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
References
- Shimada K, Iwase K, Aono T, et al. Carcinosarcoma of the gallbladder producing alpha-fetoprotein and manifesting as leukocytosis with elevated serum granulocyte colony-stimulating factor: report of a case. Surg Today 2009;39:241-6. [PubMed]
- Jonson AL, Bliss RL, Truskinovsky A, et al. Clinical features and outcomes of uterine and ovarian carcinosarcoma. Gynecol Oncol 2006;100:561-4. [PubMed]
- Batsakis JG, Suarez P. Sarcomatoid carcinomas of the upper aerodigestive tracts. Adv Anat Pathol 2000;7:282-93. [PubMed]
- Zarbo RJ, Crissman JD, Venkat H, et al. Spindle-cell carcinoma of the upper aerodigestive tract mucosa. An immunohistologic and ultrastructural study of 18 biphasic tumors and comparison with seven monophasic spindle-cell tumors. Am J Surg Pathol 1986;10:741-53. [PubMed]
- Iyomasa S, Kato H, Tachimori Y, et al. Carcinosarcoma of the esophagus: a twenty-case study. Jpn J Clin Oncol 1990;20:99-106. [PubMed]
- Sodergren MH, Silva MA, Read-Jones SL, et al. Carcinosarcoma of the biliary tract: two case reports and a review of the literature. Eur J Gastroenterol Hepatol 2005;17:683-5. [PubMed]
- Kadono J, Hamada N, Higashi M, et al. Carcinosarcoma of the extrahepatic bile duct. J Hepatobiliary Pancreat Surg 2005;12:328-31. [PubMed]
- Loud PA, Warshauer DM, Woosley JT, et al. Carcinosarcoma of the extrahepatic bile ducts: cholangiographic and CT appearance. Abdom Imaging 1997;22:85-6. [PubMed]
- Yoon GS, Choi DL. Sarcomatoid carcinoma of common bile duct: a case report. Hepatogastroenterology 2004;51:106-9. [PubMed]
- Jang KS, Jang SH, Oh YH, et al. Sarcomatoid Carcinoma of the Distal Common Bile Duct- A Case Report. Korean J Pathol 2005;39:360-3.
- Aurello P, Milione M, Dente M, et al. Synchronous carcinosarcoma of the intrapancreatic bile duct and carcinoma in situ of wirsung duct: a case report. Pancreas 2008;36:95-7. [PubMed]
- Tanaka M, Ajiki T, Matsumoto I, et al. Duodenal protrusion by carcinosarcoma of the extrahepatic bile duct. Dig Endosc 2012;24:484. [PubMed]
- Wick MR, Swanson PE. Carcinosarcomas: current perspectives and an historical review of nosological concepts. Semin Diagn Pathol 1993;10:118-27. [PubMed]
- Nomura K, Aizawa S, Ushigome S. Carcinosarcoma of the liver. Arch Pathol Lab Med 2000;124:888-90. [PubMed]
- de Brito PA, Silverberg SG, Orenstein JM. Carcinosarcoma (malignant mixed müllerian (mesodermal) tumor) of the female genital tract: immunohistochemical and ultrastructural analysis of 28 cases. Hum Pathol 1993;24:132-42. [PubMed]
- Nappi O, Glasner SD, Swanson PE, et al. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of ‘carcinosarcomas’ and ‘spindle-cell carcinomas’. Am J Clin Pathol 1994;102:331-40. [PubMed]
- Sadamori H, Fujiwara H, Tanaka T, et al. Carcinosarcoma of the gallbladder manifesting as cholangitis due to hemobilia. J Gastrointest Surg 2012;16:1278-81. [PubMed]
- Kim MJ, Yu E, Ro JY, et al. Sarcomatoid carcinoma of the gallbladder with a rhabdoid tumor component. Arch Pathol Lab Med 2003;127:e406-8. [PubMed]
- Takahashi Y, Fukushima J, Fukusato T, et al. Sarcomatoid carcinoma with components of small cell carcinoma and undifferentiated carcinoma of the gallbladder. Pathol Int 2004;54:866-71. [PubMed]
- Ajiki T, Nakamura T, Fujino Y, et al. Carcinosarcoma of the gallbladder with chondroid differentiation. J Gastroenterol 2002;37:966-71. [PubMed]
- Iwaya T, Maesawa C, Tamura G, et al. Esophageal carcinosarcoma: a genetic analysis. Gastroenterology 1997;113:973-7. [PubMed]
- Born MW, Ramey WG, Ryan SF, et al. Carcinosarcoma and carcinoma of the gallbladder. Cancer 1984;53:2171-7. [PubMed]
- Okabayashi T, Sun ZL, Montgomey RA, et al. Surgical outcome of carcinosarcoma of the gall bladder: a review. World J Gastroenterol 2009;15:4877-82. [PubMed]
- Pu JJ, Wu W. Gallbladder carcinosarcoma. BMJ Case Rep 2011;2011:bcr0520103009.
- Liu KH, Yeh TS, Hwang TL, et al. Surgical management of gallbladder sarcomatoid carcinoma. World J Gastroenterol 2009;15:1876-9. [PubMed]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 2000;232:557-69. [PubMed]
- Oh TG, Chung MJ, Bang S, et al. Comparison of the sixth and seventh editions of the AJCC TNM classification for gallbladder cancer. J Gastrointest Surg 2013;17:925-30.
- Lumsden AB, Mitchell WE, Vohman MD. Carcinosarcoma of the gallbladder: a case report and review of the literature. Am Surg 1988;54:492-4. [PubMed]
- Fagot H, Fabre JM, Ramos J, et al. Carcinosarcoma of the gallbladder. A case report and review of the literature. J Clin Gastroenterol 1994;18:314-6. [PubMed]
- Harris MA, Delap LM, Sengupta PS, et al. Carcinosarcoma of the ovary. Br J Cancer 2003;88:654-7. [PubMed]
- Kubota K, Kakuta Y, Kawamura S, et al. Undifferentiated spindle-cell carcinoma of the gallbladder: an immunohistochemical study. J Hepatobiliary Pancreat Surg 2006;13:468-71. [PubMed]
- Ariyoshi K, Kawauchi S, Kaku T, et al. Prognostic factors in ovarian carcinosarcoma: a clinicopathological and immunohistochemical analysis of 23 cases. Histopathology 2000;37:427-36. [PubMed]
- Inoshita S, Iwashita A, Enjoji M. Carcinosarcoma of the gallbladder. Report of a case and review of the literature. Acta Pathol Jpn 1986;36:913-20. [PubMed]


