Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: single center experience of 90 cases
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor worldwide and the most common primary liver cancer (1). Liver resection or liver transplantation is the therapeutic gold standards in patient with HCC with or without underlying liver disease. HCC develops in the context of cirrhotic livers in approximately 80% of cases (2). Liver resection in patients with cirrhosis have an increased risk of significant postoperative complications including ascites, gastrointestinal bleeding, encephalopathy, portal vein thrombosis and pleural effusion (3,4). Nowadays, laparoscopic liver resections (LLR) are commonly performed in patients with HCC and chronic liver disease (5). Since 2008, the Louisville consensus of experts suggested that the best indications for laparoscopy were solitary lesions less than 5 cm, located in the anterior segments, at a distance from the line of transection, the hepatic hilum, and the vena cava (6). The aim of this study was to evaluate the feasibility, morbidity and mortality of the laparoscopic approach for the resection of HCC in cirrhotic patients.
Material and methods
From 2004 to September 2014, 90 patients underwent a LLR for HCC. Data were collected in a prospectively maintained database since 2001. All patients were subject to preoperative general evaluation based on patient’s age, gender, body mass index (BMI), number of lesions, Child-Pugh score, Model for End-stage Liver Disease (MELD) score. Perioperative collected data were surgical time, need for and duration of a Pringle maneuver, blood loss, need for transfusions, Clavien-Dindo classification, length of stay. Data from histopathological examination included: nodules size and number, Edmonson grade, TNM and non-tumoral hepatic parenchyma. Preoperative evaluation was based on patient general condition and tumour biological status during our weekly multidisciplinary team (anesthesiologist, hepatologist, radiologist and surgeon) meeting. In our practice we do not routinely use indocyanine green test even in Child-Pugh B patients. The hepatic functional status was evaluated according to Belghitifu criteria (7). In cases of patients with acute alcohol related liver dysfunction the surgical procedure was postponed by 3 to 4 weeks.
The diagnoses of HCC were based on the appropriate imaging approaches including triple-phase computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound scan (US). Wedge liver resection was preferred when the lesion was superficially located. Instead a segmentectomy was performed when the tumor was deeply located. Postoperative care was performed with our hepatologist. Liver functions follow up was checked in all patients on post-operative day 1, 3 and before medical discharge. If liver failure was suspected daily blood exams and eventually abdominal CT was performed. Abdominal drainage when present was removed with clear serous fluid less than 100 cc. Bacteriological cultures was not routinely request. Patients were mobilized in first post-operative day and oral intake when bowel sound was found.
Surgical procedure
Patients were placed supine on the operative table with lower limbs apart, with the surgeon standing between the legs. Access to the abdomen was gained by open technique and pneumoperitoneum was maintained at 12 mmHg. A 10-mm port at the umbilicus housed a 30° video-camera. When preoperative radiological exams showed a periumbelical venous drainage the open access was moved from the umbilical site. The other three trocars were positioned along a semicircular line with the concavity facing the right subcostal margin. Diagnostic laparoscopy was first performed and the liver was examined using laparoscopic ultrasonography (Aloka Hitachi Medical Systems Europe Holding AG Zug, Switzerland) to exclude abdominal carcinomatosis and to confirm the extension of the HCC. Steep reverse Trendelenburg position was maintained. Central venous pressure was kept <5 mmHg during resection. Hepatic transection was performed with Enseal device (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA), clips, and application of Endo GIA vascular staples (Tyco Healthcare) to the portal pedicles when necessary. After section, all specimens were placed inside a bag and extracted following enlargement of the camera port, in cases of major resections a Pfannenstiel incision was performed. Pringle maneuver was performed when necessary. No routine drainage was used.
Results
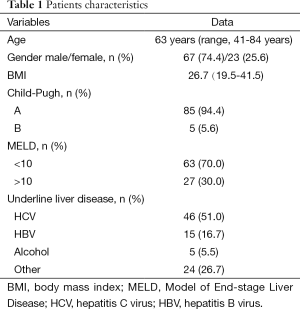
Patients’ characteristics are summarized in Table 1. Median age was 63 years; 67 (74.4%) patients were male. Median BMI was 26.7. Underlying liver disease was known in 68 patients: in 46 patients due to hepatitis C virus (HCV) infection, in 15 patients due to hepatitis B virus (HBV), in 5 patients due to previous alcohol abuse. Two patients were HIV co-infected. Child-Pugh Score was of grade A in 85 patients and of grade B in 5 patients; 63 patients had a MELD <10 and 27 patients MELD >10.
Full table
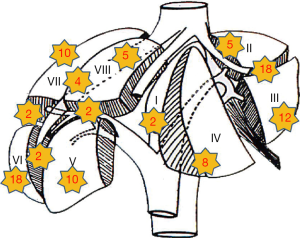
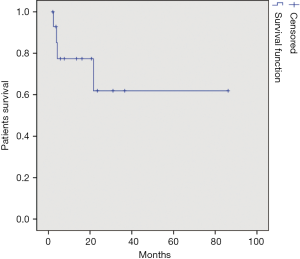
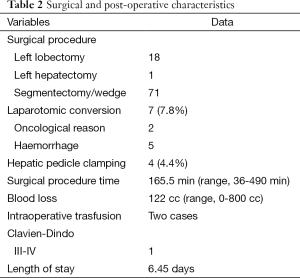
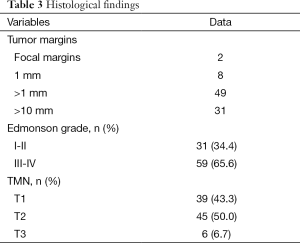
A total of 18 left lateral sectionectomies, 1 left hepatectomy and 71 wedge resections or segmentectomies were performed. Conversion to laparotomy was necessary in 7 (7.7%) patients (five cases for bleeding and two cases for oncological reasons). In four cases, hepatic pedicle clamping was required during liver resection (clamping time were 10, 11, 15 and 20 min). Clamping was performed in two cases for segment V, in one case for segment VI and in one case for a left lateral sectionectomy. In two cases a blood transfusion was required. Blood loss estimation was 122 cc (range, 0-800 cc); medium was 50 cc. Operation time was 165.5 min (range, 36-490 min); medium was 150 min. Length of stay was 6.45 days (range, 2-25 days); medium was 6 days. All surgical and post-operative characteristics are summarized in Table 2. In 90 patients, 98 HCC were resected: 79 patients had one nodule, 8 patients had two nodules and 1 patient had three nodules. HCC nodule medium diameter was 29 mm (range, 4-100 mm); medium was 25 mm. Tumor margins distance was 16 mm (range, 0-35 mm) with median 5 mm. In two cases tumor margin was 0 mm, in eight cases margin was 1 mm. Histological findings are shown in Table 3. Tumor localization is represented in Figure 1, 70 nodules were localized within the anterior sectors and 28 nodules within the posterior sectors. Patients survival at 1, 3 and 5 years was respectively of 87, 93%; 34, 48% and 13, 79% (Figure 2). However, 47 patients were treated in the last 3 years so we have not enough follow up for those patients. If we consider only the patients treated before 2012 survival rates are respectively of 84, 61%; 61, 53% and 30, 76%.
Full table
Full table
Discussion
This study demonstrates the feasibility of a laparoscopic approach in HCC patients with chronic liver disease, even in selected Child B patients. LLR is now accepted worldwide, considering the excellent results shown in specialized centers (8,9). The first consensus conference of LLR surgeons take place in Louisville in 2008; since that conference surgical indications continued to evolve. Liver function is considered an important indication for surgery. Most centers reserve surgery for patients with Child-Pugh Class A and consider those with Child-Pugh Class B-C for transplantation (5,9). We recently commented on a review comparing Middle Eastern and Western countries experiences (5,10) reporting a cumulative experience of 109 resections in class B-C patients. In our series LLR could be safely performed in selected Child B patients.
The main clinical advantage of LLR is the significantly lower rate of postoperative complications considering that the abdominal wall is preserved and kinetics of the diaphragm are improved (11). The long skin incision in open surgery may induce several disadvantages for patients. Less post-operative ascites has been suggested to be a consequence of better collateral venous drainage due to less liver mobilization in LLR. In our series, 1.1% of complications were classified as Clavien-Dindo > II. Blood transfusions were required in 2% of cases, results which are in line with others experiences (12). The haemostatic effect of the pneumoperitoneum associated with the image magnification has been emphasized to reduce blood losses during LLR (13).
Patients undergoing liver transplantation after a previous LLR have shorter operation times and lower blood losses. Laurent et al. suggest preferring LLR over open surgery in potential transplant candidates (14). Treatments of recurrence (resections and transplantation) were facilitated in patients previously treated with LLR.
No significant difference in recurrence-free or overall survival between open or laparoscopic approach was described (12). As suggest by Shi et al.’s works, marginal resection did not negatively affect postoperative recurrence-free survival (15). In our experience medium tumor margin distance was 16 mm. However we recommend the systematic use of intraoperative ultrasound to correctly locate the tumor and to keep the planned margin, as advocated by Ferrero et al. (16).
A recent meta-analysis concluded that LLR for HCC is superior to open approach in terms of its perioperative results and does not compromise the oncological outcomes (17).
Belli et al. confirmed in a series of 65 highly selected patients the feasibility of LLR of HCC in patients with liver cirrhosis (18). Kanazawa et al. suggest that even in cases of recurrent HCC, LLR is a safe and feasible procedure reducing intraoperative blood loss, ascites, major complications and consequently shortens the length of stay (19). Some authors have compared the laparoscopic vs. open approach for HCC in cirrhotic livers and concluded that laparoscopic approach should be considered as standard care, considering the minimally invasive approach as safe and potentially providing better outcomes compared to the open approach (20,21).
Conclusions
LLR for HCC should be performed by dedicated hepatobiliary and laparoscopic surgeons. The use of LLR in cirrhotic patients is proposed by many centers as the first-line of treatment for HCC or as a bridge treatment to liver transplantation.
Acknowledgements
We thank Gabriele Spoletini, MD, who provided English language editing for our manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethical committee.
References
- Lai Q, Lerut JP. Hepatocellular cancer: how to expand safely inclusion criteria for liver transplantation. Curr Opin Organ Transplant 2014;19:229-34. [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [PubMed]
- Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210-5. [PubMed]
- Kanazawa A, Tsukamoto T, Shimizu S, et al. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc 2013;27:2592-7. [PubMed]
- Ettorre GM, Levi Sandri GB. Laparoscopic approach for hepatocellular carcinoma: where is the limit? Chin J Cancer Res 2014;26:222-3. [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [PubMed]
- Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46. [PubMed]
- Ettorre GM, Laurenzi A, Lionetti R, et al. Laparoscopic liver resections in normal and cirrhotic livers: a retrospective analysis in a tertiary hepato-biliary unit. Dig Liver Dis 2014;46:353-7. [PubMed]
- Herman P, Perini MV, Coelho FF, et al. Laparoscopic resection of hepatocellular carcinoma: when, why, and how? A single-center experience. J Laparoendosc Adv Surg Tech A 2014;24:223-8. [PubMed]
- Piardi T, Sommacale D, Baumert T, et al. Laparoscopic resection for hepatocellular carcinoma: comparison between Middle Eastern and Western experience. Hepatobiliary Surg Nutr 2014;3:60-72. [PubMed]
- Lai EC, Tang CN, Yang GP, et al. Multimodality laparoscopic liver resection for hepatic malignancy--from conventional total laparoscopic approach to robot-assisted laparoscopic approach. Int J Surg 2011;9:324-8. [PubMed]
- Gaillard M, Tranchart H, Dagher I. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol 2014;20:4892-9. [PubMed]
- Aldrighetti L, Guzzetti E, Pulitanò C, et al. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 2010;102:82-6. [PubMed]
- Laurent A, Tayar C, Andréoletti M, et al. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2009;16:310-4. [PubMed]
- Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36-43. [PubMed]
- Ferrero A, Lo Tesoriere R, Russolillo N, et al. Ultrasound-guided laparoscopic liver resections. Surg Endosc 2015;29:1002-5. [PubMed]
- Zhou YM, Shao WY, Zhao YF, et al. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci 2011;56:1937-43. [PubMed]
- Belli G, Fantini C, Belli A, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhosis: long-term outcomes. Dig Surg 2011;28:134-40. [PubMed]
- Kanazawa A, Tsukamoto T, Shimizu S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2013;20:512-7. [PubMed]
- Twaij A, Pucher PH, Sodergren MH, et al. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol 2014;20:8274-81. [PubMed]
- Memeo R, de’Angelis N, Compagnon P, et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg 2014;38:2919-26. [PubMed]