Comparison of high-intensity focused ultrasound therapy and radiofrequency ablation for recurrent hepatocellular carcinoma
Hepatocellular carcinoma (HCC), which accounts for more than 90% of primary liver cancer, is a major health problem worldwide, and is the third most common cause of cancer-related death. It is the fifth most prevalent cancer in men and the seventh in women (1-3). The prognosis for untreated HCC is generally poor, and curative treatments consist of surgical resection, radiofrequency ablation (RFA), and liver transplantation (1-3).
Technical advances in surgery for HCC have improved the survival of HCC patients considerably during recent years. However, only 20% of HCC patients are amenable to surgical resection on presentation (4-6). Locoregional ablative therapies, such as RFA, percutaneous microwave ablation, cryoablation, transcatheter arterial chemoembolization (TACE), and percutaneous ethanol injection offer good alternatives to resection for HCC patients (7).
RFA therapy, an alternative modality to percutaneous ethanol injection, has been widely used as a curative treatment for HCC. Currently, RFA is considered the most promising locoregional treatment for HCC. This modality induces coagulative necrosis and tissue desiccation by delivering high-frequency alternating current via electrodes placed within tissues (7-9). RFA therapy for HCC is primarily accomplished by a percutaneous approach, although open laparoscopic or thoracoscopic approaches can also be used (7-9). RFA provides a valuable treatment option for unresectable HCC. As advances in RFA therapy for HCC continue to be made, it is gradually being performed in patients with resectable HCC, as well as in our country (Japan) (8,9). In addition, RFA is a repeatable procedure because it is less invasive than surgical resection, and it can be safely performed in elderly patients with potentially comorbid diseases (7,9,10). However, there are several limitations associated with RFA for HCC despite its many potential favorable effects. These limitations include a limited ablation volume, technical limitations, expected complications dependent on tumor location, the heat sink effect, and tumor seeding (11,12).
High-intensity focused ultrasound (HIFU) ablation is an extracorporeal noninvasive ablation mode using focused ultrasound energy, which is capable of causing coagulative necrosis of the targeted HCC via intact skin without the need for surgical incision or insertion of instruments (13,14). This ablation uses a unique frequency of ultrasound waves of 0.8-3.5 MHz, which can be focused at a distance from the therapeutic transducer (13,14). HIFU can provide a potential therapeutic method for the precise ablation of entire liver tumors without damaging vital structure. HIFU also offers the first completely non-invasive approach for HCC and is therefore a promising locoregional treatment modality. Recently, HIFU has been receiving increasing interest for the management of liver tumors (13-15). However, at present, data on the long-term outcome of this treatment are limited. There have been several reports regarding the comparison between TACE plus HIFU and TACE (Table 1) (13,16,17), and whether HIFU obtains a survival benefit similar to that of RFA for patients with HCC remains unclear.
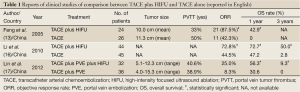
Full Table
In this issue of Annals of Surgery, Chan et al. demonstrated in their retrospective comparative study, including patients with recurrent HCC, that with a median follow-up period of 27.9 months, the 1-, 2-, and 3-year disease-free survival rates were 37.0%, 25.9%, and 18.5%, respectively, for the HIFU group, and 48.6%, 32.1%, and 26.5%, respectively, for the RFA group (P=0.61). Additionally, the 1-, 2-, and 3-year overall survival rates were 96.3%, 81.5%, and 69.8%, respectively, for the HIFU group, and 92.1%, 76.1%, and 64.2%, respectively, for the RFA group (P=0.19). There was no hospital mortality in the HIFU group, whereas two deaths occurred in the RFA group. They concluded that using HIFU for recurrent HCC is safe and promising. Although their study was retrospective in nature and had a small sample size, it appears to be a novel and well-characterized study. In their article, they also described the following three advantages of HIFU therapy compared with RFA: (I) extracorporeal conformal therapy of HIFU, indicating no surgical exposure of this therapy; (II) tumor seeding along the needle tract, which often occurs in RFA therapy for HCC, is unlikely to occur and (III) avoidance of targeted tumor puncturing.
In terms of treatment efficacy of HIFU, Chan et al. reported that the complete ablation rate was greater than 80% in the HIFU group, which is slightly lower than that of RFA in previous reports (8,9,18). This may due to the small number of patients in whom they performed HIFU therapy. With sufficient experience in clinical practice for HIFU therapy, the results of treatment efficacy of HIFU for HCC will improve. Notably, the rate of procedure-related morbidity in the HIFU group tended to be lower than that in the RFA group in their study [2 (7.4%) out of 27 patients in the HIFU group vs. 25 (22.4%) out of 76 patients in the RFA group, P=0.06] and the hospital mortality rate was 0% in the HIFU group. Their results indicated that HIFU therapy for recurrent HCC was a safe procedure. In Japan, the proportion of elderly patients with HCC and their average age is increasing. In general, elderly patients have a high incidence of comorbid diseases and are considered high-risk patients for treatment-related complications (10). Safety in HCC therapy is an essential issue, as well as treatment efficacy.
In view of previous studies regarding HIFU for HCC, there are some difficulties that need to be overcome before HIFU can be used in everyday clinical practice (19). The main limitation to clinical application of HIFU is the fact that ablation of large tumors is still time consuming [median total operating time: 151 min (range, 24-360 min)], as reported by Chan et al. in this issue of Annals of Surgery (15,19,20). In contrast, the duration of a single ablation of RFA is approximately 12 min for the 3-cm electrode of the cool-tip needle (Radionics, Burlington, MA, USA) (8). This may be problematic, especially in patients with a poor physical condition. With technical improvement, the treatment time of HIFU could be gradually reduced in the future. Another challenge in HIFU for HCC therapy is the difficulty in targeting and monitoring because the liver is subject to respiratory movements. The motion of the liver can cause misdirected ultrasound energy, which could potentially result in damage of normal tissue and incomplete tumor ablation. In addition, in cases of HCCs located just behind the ribs, ultrasound energy cannot be easily transmitted through the overlying bone structures (20). Reflection of ultrasound beams by the ribs may cause damage to the bone and adjacent liver tissue (14,20). Therefore, novel technologies in HIFU therapy for HCC should be investigated to overcome these problems.
In conclusion, although there are several difficulties in HIFU therapy for HCC in clinical practice, Chan et al. showed in their comparative study of HIFU and RFA that HIFU therapy for recurrent HCC is a safe and promising procedure. With further technical advances, this treatment can be a first line non-invasive ablative therapy for unresectable HCC. Further clinical evidence of this therapy is expected.
Acknowledgements
Disclosure: The authors have not received any financial support for this editorial and have no conflicts of interest to declare.
References
- Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg 2011;100:22-9. [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73.e1.
- de Lope CR, Tremosini S, Forner A, et al. Management of HCC. J Hepatol 2012;56:S75-87. [PubMed]
- Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol 1993;9:298-304. [PubMed]
- Zhou WP, Lai EC, Li AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg 2009;249:195-202. [PubMed]
- Nishikawa H, Arimoto A, Wakasa T, et al. Effect of transcatheter arterial chemoembolization prior to surgical resection for hepatocellular carcinoma. Int J Oncol 2013;42:151-60. [PubMed]
- Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology 2010;78:113-24. [PubMed]
- Nishikawa H, Inuzuka T, Takeda H, et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: a proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol 2011;46:1418-26. [PubMed]
- Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2011;98:1210-24. [PubMed]
- Nishikawa H, Osaki Y, Iguchi E, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: clinical outcome and safety in elderly patients. J Gastrointestin Liver Dis 2012;21:397-405. [PubMed]
- Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg 2009;249:20-5. [PubMed]
- Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010;52:762-73. [PubMed]
- Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235:659-67. [PubMed]
- Ng KK, Poon RT, Chan SC, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg 2011;253:981-7. [PubMed]
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol 2010;195:W245-52. [PubMed]
- Li C, Zhang W, Zhang R, et al. Therapeutic effects and prognostic factors in high-intensity focused ultrasound combined with chemoembolisation for larger hepatocellular carcinoma. Eur J Cancer 2010;46:2513-21. [PubMed]
- Cui L, Liu XX, Jiang Y, et al. Comparative study on transcatheter arterial chemoembolization, portal vein embolization and high intensity focused ultrasound sequential therapy for patients. Asian Pac J Cancer Prev 2012;13:6257-61. [PubMed]
- Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-30. [PubMed]
- Seinstra BA, van Delden OM, van Erpecum KJ, et al. Minimally invasive image-guided therapy for inoperable hepatocellular carcinoma: What is the evidence today? Insights Imaging 2010;1:167-81. [PubMed]
- Shen HP, Gong JP, Zuo GQ. Role of high-intensity focused ultrasound in treatment of hepatocellular carcinoma. Am Surg 2011;77:1496-501. [PubMed]


