Pancreatic cancer and thromboembolic disease, 150 years after Trousseau
Historical remarks
The Manchester surgeon Charles White first demonstrated in 1784 that “milk leg” was not caused by retained milk or lochia, but rather by obstructing clots in the veins. In 1847, the German Rudolph Virchow [1821-1902] observed that venous thrombi often migrated to the lungs (1). In 1865, the French physician Armand Trousseau [1801-1867] described that migratory venous thromboses occurring during the course of his own pancreatic cancer (2). It has after that for a long time been considered a “truth” that carcinoma of the pancreas has an inherent and unique ability to induce a hypercoagulable diathesis that leads to clinically significant thrombosis. This has, however, later on been challenged and there have been voices stating that the relationship between cancer of the pancreas and thromboembolic disorders should be de-emphasized since it is neither unique nor especially in association with pancreatic carcinoma, and since it may be almost as frequently encountered in other visceral malignancies (3). A problem with dealing with this issue is the semantics. For example, an analysis by Sack and colleagues in 1977 (4) extended the term Trousseau’s syndrome to include chronic disseminated intravascular coagulopathy associated with microangiopathy, verrucous endocarditis, and arterial emboli in patients with cancer, often occurring with mucin-positive carcinomas. After that the term has also been ascribed to other clinical situations, ranging all the way from the classic descriptions to any kind of coagulopathy occurring in the setting of any kind of malignancy. These multiple definitions of Trousseau’s syndrome are probably partly a reflection of multiple pathophysiologic mechanisms that apparently contribute to the hypercoagulability associated with cancer. Even the classic syndrome probably represents a spectrum of disorders, ranging from exaggerated fluid-phased thrombosis dependent on prothrombotic components, such as TF to a platelet- and endothelium-based selectin-dependent microangiopathy associated with mucin-producing carcinomas, along with thrombin and fibrin production (5).
We have scrutinized the current literature (since the year 2000) on “pancreatic disease and venous thrombosis” in order to find out if we today know more on the issue than Armand Trousseau did 150 years ago and if there are scientific reasons to believe that pancreatic cancer has a closer connection to thromboembolism than that seen in other malignancies.
Venous thrombosis in acute pancreatitis
Thrombosis of the legs
Phlegmasia cerulea dolens is a severe form of deep venous thrombosis with proximal occlusion in an extremity, most frequently in the ileofemoral area. This form of thromboembolic disease is rare, occurring most frequently in the terminal stage of malignancies. There is increasing evidence that pancreatitis-induced endothelial dysfunction is one of the critical pathophysiological manifestations for venous thrombosis in patients with severe form of acute pancreatitis. The diagnosing of phlegmasia usually causes no problems as the proper diagnosis is usually revealed during clinical examination and confirmed by imaging techniques. A case of phlegmasia cerulean dolens was reported as a complication of a severe form of acute hemorrhagic-necrotizing pancreatitis (6).
Portal vein thrombosis
Splanchnic vein thrombosis in pancreatic diseases may involve the portal vein, splenic vein and superior mesenteric vein, either alone or in combination. In one series of 127 consecutive patients admitted with acute pancreatitis, 20 patients (16 percent) had splanchnic venous thrombosis (7). In all cases the thrombosis was associated with a severe attack of acute pancreatitis, i.e., most of the patients with severe attacks had thrombosis, whereas none of those with mild pancreatitis. Splanchnic vein thrombosis is thus not an uncommon observation in severe AP and might be associated with pancreatic necrosis and peripancreatic collections. Recanalization has been observed in almost a third of patients, irrespectively of whether they receive systemic anticoagulation or not. It seems fair, however, to state that the high incidences do not reflect the “true” incidence of splanchnic or splenic vein thrombosis that would be found in an unselected series of patients with acute pancreatitis. Nonetheless, it can be assumed that in a referral centre for patients with severe acute pancreatitis, this complication will be encountered more frequently than usually expected.
Pulmonary embolism
Pulmonary embolism is according to the “old” literature due to embolism of a thrombus (blood clot) from the deep veins in the legs, but there are today also indications that thrombosis can start in the auriculae of the heart. Untreated, pulmonary embolism has a mortality rate of approximately 30 percent—maybe higher if the patient has a severe acute pancreatitis in combination with the pulmonary embolus. Still, pulmonary embolism in acute pancreatitis has been reported to be rare, maybe as these patients today usually are given preventive low-molecular heparinoids. A case of pulmonary embolism with acute pancreatitis was recently presented (8).
Occlusion of peripancreatic veins in chronic pancreatitis
The course of chronic pancreatitis has been characterized, including the rate of venous thrombosis. In a retrospective study, 118 patients with chronic pancreatitis were included (9). Thirty percent of patients had surgery, 74 percent within 5 years after diagnosis. The incidence of peripancreatic venous thrombosis was high. Occlusions of peripancreatic veins were observed more often in patients with alcoholic than in idiopathic etiology (38 vs. 17 percent). Other evaluated parameters did not differ in comparison to the literature. It was concluded that occlusion of veins in chronic pancreatitis was observed more often than previously reported, but if the incidence should be calculated accurately it must be studied prospectively with defined diagnostic procedures.
Portal vein thrombosis
One hundred and seventy chronic pancreatitis patients undergoing surgery were included in another study where the average duration of the chronic pancreatitis was 12 years (10). Six patients developed portal vein thrombosis giving a prevalence of 3.5 percent. Symptoms included low protein ascites (2), significant weight loss (5), splenomegaly (4), and segmental hepatic necrosis (1). Mesenteric vein was not involved by thrombus in any patient, while splenic vein thrombosis was present in all patients. None of the patients had bleeding from esophageal-gastric varices during 4 years mean follow-up. Risk factors for portal vein thrombosis were alcoholic etiology, chronic pancreatitis duration more than 10 years, recent acute episodes of acute pancreatitis, infected fluid collections and previous splenic vein thrombosis. Portal vein thrombosis usually evolved to cavernoma, which makes surgery more difficult and risky.
Portal venous occlusion may progress to extrahepatic generalized portal hypertension. Pancreatic head resections have generally been regarded risky or contraindicated in patients with chronic pancreatitis and extrahepatic generalized portal hypertension. However, it was recently reported that pancreatic head resection may be performed in patients with extrahepatic generalized portal hypertension with an acceptable safety, especially when performing preoperative interventional recanalization of portal vein thrombosis by restoring normal splanchnic blood flow (11).
Isolated splenic vein thrombosis
Isolated splenic vein thrombosis leading to sinistral (left-sided) portal hypertension and variceal bleeding is an unusual complication of chronic pancreatitis. Recent improvements in cross-sectional imaging have led to the identification of splenic vein thrombosis also in patients with minimal symptoms. Previous studies have suggested that splenic vein thrombosis results in a high likelihood of gastric variceal bleeding, and that splenectomy should be performed to prevent hemorrhage (12-14). However, recent studies have found that gastric variceal bleeding from pancreatitis-induced splenic vein thrombosis is rather uncommon (4-12 percent) and therefore routine splenectomy is not recommended in asymptomatic patients (15,16).
Transcapsular collateral veins
It has been reported that transcapsular collaterals frequently occur in patients with chronic portal vein thrombosis due to hepatobilary surgery or severe pancreatitis. They are associated with ectopic varices; therefore, awareness of transcapsular collaterals in this patient subgroup will help to localize ectopic varices as potential bleeding source (17).
Venous thrombosis in pancreatic cancer
In 1865, Trousseau first described the association between venous thrombosis and malignancy. It is since then repeatedly described that unprovoked deep vein thrombosis of the legs precedes the diagnosis of malignancy in at least 7 percent of these cases. In bilateral deep vein thrombosis the risk of occult malignancy exceeds 40 percent (18).
Cancer is one of the most important acquired—but often overlooked—risk factor for the development of venous thromboembolism (VTE). Tumors can express procoagulant proteins, for example, and tumor masses may compromise venous blood flow by extrinsic compression of adjacent low-flow vessels. Cancers can also induce the production of inflammatory cytokines that indirectly contribute to the development of hypercoagulability and the risk of thromboembolism. Additional risk factors for VTE experienced by patients with cancer include immobilization, because of cancer or its treatment, and the potential presence of thrombophilic genetic factors. Many common therapeutic modalities also increase VTE risk, including surgery, chemotherapy, adjuvant hormonal manipulation, the use of angiogenesis inhibitors, and the presence of central venous access devices. The risk of VTE seems to be greater with certain tumor types, such as cancers of the pancreas, kidney, or brain (19).
Superficial and deep venous thrombosis
In contrast to deep venous thrombosis and pulmonary embolism, superficial venous thrombosis has usually not been considered to be a marker of occult cancer. However, actual data regarding that hypothesis have been limited. In a Danish population-based health registry (20), it was shown that venous thrombosis (also in superficial veins), whenever it is seen in the lower limbs, may be a preclinical marker of prevalent cancer, particularly during the first year after diagnosis, but is not specifically indicating a pancreatic cancer.
Pulmonary embolism
The incidence of pulmonary embolism in oncology outpatients has been estimated at 2.9 percent (21). The incidence was highest in tumors of the central nervous system, hepatobiliary, pancreatic (5.8 percent), and upper gastrointestinal tract. The risk of pulmonary embolism was significantly higher for central nervous system, pancreatic (OR 2.15), upper gastrointestinal, and lung/pleural malignancies.
Risk factors for venous thromboembolism (VTE)
Investigations of incidence in patients in hospital
Recent epidemiological data support the empirical observation of Trousseau that digestive cancer may induce deep vein thrombosis. There are many studies presented on this issue. The epidemiology of thromboembolism in cancer patients was for example investigated by linking the California Cancer Registry database to the discharge records of all patients hospitalized in California public hospitals (22). Nineteen types of cancer were studied. The incidence of VTE was highest in patients who presented with metastatic cancer, particularly clinically aggressive cancers associate with a high one-year mortality rate, such as pancreatic cancer.
In another study, the frequency, risk factors, and trends associated with VTE were examined among hospitalized cancer patients using an American discharge database (23). This included 1,824,316 hospitalizations at 133 US medical centers. Among 1,015,598 cancer patients, 34,357 (3.4 percent) were diagnosed with deep venous thrombosis and 11,515 with pulmonary embolism (1.1 percent) for an overall VTE rate of 4.1 percent. The site of cancer with the highest rates of VTE was pancreas (Table 1).
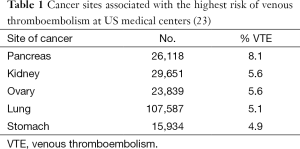
Full table
The medical histories of 409 patients with different tumors were analyzed for the frequency of venous thrombosis with or without pulmonary embolism (24). Venous thrombosis was most often seen in metastasizing tumors and in colorectal carcinoma (40 percent), hematological system diseases (29 percent), gastric cancer (30 percent), bronchial, pancreas and ovarian carcinoma (29 percent), and carcinoma of the prostate (17 percent). It must though be emphasized that all incidence and prevalence figures for thromboembolism—at least in cancer patients—should be prospectively based if used for scientific purposes.
Population based studies
By another Danish group incidence rates (IRs) for VTE hospitalization in a cohort of cancer patients (n=57,591) and in a comparison general-population cohort (n=287,476) were investigated (25). Throughout the follow-up, VTE IR was higher among the cancer patients (IR =8.0) than the general population (IR =4.7), particularly in the first year after cancer diagnosis. IRs of VTE were highest in patients with pancreatic cancer (IR =40.9), and among patients with advanced-stage cancers (IR =27.7) or those who received chemotherapy or no/symptomatic treatment. Also the adjusted relative risks (RRs) for VTE were highest among patients with pancreatic cancer (adjusted RR =16.3).
Metastatic cancer
Several recent studies have shown that the incidence of VTE is highest in patients who present with metastatic cancer, particularly cancers such as pancreatic cancer (26). The IR of VTE is highest in the first few months after the diagnosis of cancer, and it decreases over time thereafter. Active chemotherapy, the use of erythropoietin agents, and the use of certain anti-cancer therapies such as thalidomide, high-dose steroids, and anti-angiogenic therapy also increase the risk of thrombosis. Similar to patients without cancer, the risk of VTE is higher in patients with coexisting chronic medical illnesses. Development of VTE is clearly associated with decreased survival and this effect is greater among patients initially diagnosed with local or regional stage cancer compared to patients with metastatic cancer.
An early warning sign?
It has for many years been speculated that deep vein thrombosis or pulmonary embolism could represent a warning sign for a latent cancer. The practical question about this association is: shall we recommend searching for pancreatic and other cancers in all patients with thrombosis? Present data show that the strategy to look for such malignancies in patients with thrombosis on a routine base is not cost-effective (27).
Modern diagnostics
The correct diagnosis of tumor thrombosis and its differentiation from VTE can alter patient management and prevent unnecessary long-term anticoagulation treatment. It appears that PET/CT may be helpful in the diagnosis of occult tumor thrombosis and its differentiation from VTE (28).
Biological factors
Genetics
In recent years it has been suggested that genes involved in neoplastic transformation may acts as determinants of cancer coagulopathy (Figure 1). Genes frequently reported to be regulators of cancer coagulopathy include activation of oncogenes such as KRAS, c-MET and inactivation of tumor suppressor genes such as p53 (29,30). From a biological point of view, it is believed that activation of the coagulation cascade and the production of the fibrin provides the cancer cells with a scaffold for anchorage and invasion, while coagulation proteins also promote mitogenic and oncogenic signals of importance for tumor invasion and progression (29).
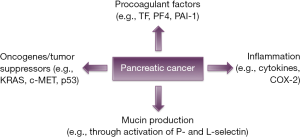
Tissue factor (TF)
Hemostatic activation is common in pancreatic cancer and may be linked to angiogenesis and VTE. The primary initiator of coagulation, the transmembrane receptor TF, has gained considerable attention as a determinant of tumor progression. It has been reported that TF expression occurs early in pancreatic neoplastic transformation and is associated with vascular endothelial growth factor (VEGF) expression, increased microvessel density, and possibly clinical VTE in pancreatic cancer (31).
On complex formation with its ligand, coagulation factor VIIa, TF influences protease-activated receptor-dependent tumor cell behavior, and regulates integrin function, which facilitate tumor angiogenesis both in vitro and in mouse models. Furthermore, evidence exists that an alternatively spliced isoform of TF also affects tumor growth and tumor angiogenesis. In patient material, TF expression and TF cytoplasmic domain phosphorylation correlate with disease outcome in many, but not in all, cancer subtypes, suggesting that TF-dependent signal transduction events are a potential target for therapeutic intervention in selected types of cancer (32). It may be speculated on anticancer therapy by targeting TF.
Platelet factor 4 (PF4)
PF4 has been proposed also as a diagnostic biomarker for pancreatic cancer. High PF4 serum levels have been associated with an increased risk for the development of VTE and poor survival (33).
Plasminogen activator inhibitor type 1 (PAI-1)
PAI-1 is a key inhibitor of fibrinolysis. Heightened PAI-1 activity causes a reduction in fibrinolytic activity and increases the risk of thrombosis (34). In a previous study, we reported that peaks in plasma PAI-1 may be associated with thrombotic events in patients with pancreatic cancer (35).
Mucins
Mucins are high-molecular-weight glycoproteins that have O-glycosylated tandem repeat region rich in proline, threonine and serine residues. Pancreatic cancer is associated with aberrant expression and glycosylation of both transmembrane and secreted mucins. This aberrant expression of mucins has been found to facilitate pancreatic tumor growth, invasiveness and subsequent metastasis (36). Trousseau’s syndrome often occurs with mucinous adenocarcinomas and it is believed that selectin-mucin interactions might trigger this event. In vivo analysis have shown that injection of carcinoma mucins into mice generates platelet-rich microthrombi dependent on P- and L-selectin but not thrombin (37,38).
Inflammation
Inflammatory processes are well known to impact on the fibrinolytic system and to promote thrombosis and cancer. Tumor cells produce and secrete a number of different proinflammatory cytokines, which can contribute to the hypercoagulable state of malignancy. Of note, TNF-α and IL-1 can induce upregulation of TF and downregulation of thrombomodulin, a cofactor for thrombin binding that mediates protein C activation and inhibits thrombin activity (39,40). Other proposed mechanisms involve the overexpression of cyclooxygenase-2 (COX-2), which can induce a pro-thrombotic state (41).
Impact of thrombosis on outcome
The natural history of splanchnic venous thrombosis in advanced pancreatic cancer is poorly characterized. It has been found that progressive splanchnic venous thrombosis in advanced pancreatic cancer is associated with an increased rate of complications and poor survival (42). Anticoagulants have been shown to be safe and may reduce the risk of splanchnic venous thrombosis-related ascites but their impact on survival remains uncertain.
Several studies have analyzed the risk of VTE associated with pancreatic cancer and its consequences on treatment and survival. While most studies suggest that VTE may reflect the presence of a biologically more aggressive cancer that in turn leads to a worse prognosis (43-46), others suggest that VTE has little effect on survival (47,48).
Postoperative venous thrombosis after pancreatic resection
Postoperative thrombotic occlusion of the portal vein is a serious complication after pancreatectomy. Despite the increased theoretical risk for thrombosis formation, there are few studies that have estimated the incidence of thrombosis in the portal system after pancreatic resections.
In a previous study, we investigated the occurrence of portal venous system thrombosis after pancreatic resection, and found an incidence of 3.5 percent (49). The higher incidence thrombosis in the portal system after pancreatic resections with total pancreatectomy compared with right-sided resection is intriguing and raises the question whether this subgroup requires more active surveillance.
Vascular injury or portal vein reconstruction during surgery increases the risk of postoperative thrombosis formation. One late portal vein thrombosis was reported among 18 patients undergoing Whipple’s procedure complicated by vascular injury (50). Among 64 patients undergoing pancreaticoduodenectomy with venous reconstruction (51), one study reported a 17 percent incidence of portal vein thrombosis, of which 5 percent were acute (within 30 days). In a similar study, portal vein thrombosis was identified in 6 (21 percent) of 28 patients who underwent pancreaticoduodenectomy with portal vein reconstruction (52).
The incidence of pulmonary embolism has been estimated at 7.3 percent and deep venous thrombosis at 1.3 percent after pancreatic resection (53). The discrepancy between pulmonary embolism and deep venous thrombosis incidence in the study shows that one should question the relationship between pulmonary embolism and deep venous thrombosis. Pulmonary embolism might occur de novo. In that case this has implications for thrombosis treatment and particularly the possible usefulness of vena cava filters.
Treatment of venous thrombosis in pancreatic disease
Treatment options
A significant proportion of cancer-associated VTE occurs in the ambulatory setting and is associated with poorer outcomes and reduced survival. Risk for VTE is influenced by patient, cancer and treatment-specific factors. Recent studies have identified biomarkers associated with increased VTE risk in malignancy, including leukocyte and platelet counts, TF, prothrombin split products, D-dimer, P-selectin, factor VIII and C-reactive protein. Recent and ongoing clinical trials have focused on VTE prophylaxis with low molecular weight heparins (LMWHs) in high-risk cancer outpatients, particularly those with pancreatic cancer. These studies have yielded encouraging preliminary results but whether thromboprophylaxis provides significant benefit to unselected cancer outpatients remains unclear. A risk stratification model incorporating known risk factors and biomarkers can identify those patients at highest risk (54).
Treatment results
Emerging clinical data strongly suggest that anticoagulant treatments may improve cancer patient survival by decreasing thromboembolic complications as well as by anticancer effects (55). There are authors claiming that patients with pancreatic cancer, as with other visceral cancers, should be submitted to a prophylactic strategy to prevent thrombosis: therapy with LMWH for several weeks and that it was beneficial in several trials (27).
There are authors also claiming that if primary thromboprophylaxis of cancer patients is considered, treatment should begin immediately after cancer diagnosis, and it should be targeted toward patients who have a biologically aggressive cancer that is initially metastatic and/or toward patients who have several chronic co-morbid conditions. Secondary thromboprophylaxis should be targeted toward patients who have evidence of an ongoing active malignancy (22).
Resolution rate
Recurrent VTE rates of 9-17 percent occur (56) despite the use of therapeutic anticoagulation. LMWHs afford several advantages over warfarin. Nevertheless, even with LMWHs more than 20 percent of patients have VTE propagation. Complete resolution and partial resolution of DVTs occurred in one study in up to 38 and 54 percent, respectively, after 6 months of anticoagulation (57,58), and thrombi remain detectable in half of non-cancer patients after a year (59). Recently, Factor Xa inhibitors have become available and require further study to determine if they offer therapeutic improvements in this patient population. As part of the CAT (Cancer and Thrombosis) trial, complete resolution rates with the Factor Xa inhibitor fondaparinux sodium were prospectively evaluated. The results showed that complete resolution rate of VTE with fondaparinux was among the highest reported in patients with cancers (including pancreatic cancer), and occurred within 8 weeks of starting anticoagulation (60).
Low-molecular-weight heparin
Given the likelihood that the majority of patients with splanchnic vein thrombosis complicating acute pancreatitis will be asymptomatic and the reported spontaneous recanalization rate is 30 per cent, it would seem sensible to reserve anticoagulation therapy for patients with progression of thrombosis; however, the evidence base for this remains poor (7). It would seem important to try and identify those who are at a higher risk of bleeding particularly if anticoagulation is being considered. Careful attention to the drainage of the coronary vein may help select those at an increased risk of esophageal varices. Routine endoscopy, which is the gold standard to identify esophageal varices, should also be considered. It should also be appreciated that splenomegaly is not present in all patients with splenic vein thrombosis and hence not a reliable marker of sinustral hypertension. Thought should also be given to the presence of cirrhosis which may contribute to portal hypertension and be associated with an increased risk of bleeding (15).
One retrospective analysis aimed to identify whether LMWHs might improve survival in patients receiving chemotherapeutic treatment for advanced pancreatic cancer (61). It was found that patients receiving LMWHs had a survival advantage. Nevertheless, the observations need confirmation by prospective randomized studies.
A recently published randomized prospective trial showed significant reduction in the incidence of VTE with the use of prophylactic dalteparin in pancreatic cancer patients receiving gemcitabine (62). Results of ongoing studies will reveal whether thromboprophylaxis could potentially improve survival in pancreatic cancer.
The results from CONKO-004 were reported in abstract form (63). In this multicenter prospective randomized phase III trial of patients with advanced pancreatic cancer, the patients were randomized to chemotherapy with or without primary prophylaxis enoxaparin. The intention-to-treat analysis showed a significant decrease in symptomatic thrombosis without any difference in major bleeding events. However, this trial closed early after a data and safety monitoring committee decision confirming the early significant thrombosis reduction, and is therefore underpowered to observe a survival benefit between the arms.
Vena cava filter
The efficacy and safety of inferior vena cava filter addition to parenteral anticoagulation in treating cancer patients with VTE remains controversial and no randomized trials have been conducted in this population. A prospective randomized, controlled, trial was initiated to determine if the addition of inferior vena cava filter placement was advantageous in cancer patients (64). While a trend toward lessened survival was seen in the inferior vena cava filter and parenteral anticoagulation arm, it is difficult to say that this is treatment related due to the heterogeneous patient population. With the cost and invasiveness of inferior vena cava filter placement and without a benefit in recurrent pulmonary embolism rate or safety, inferior vena cava filter should not routinely be added to anticoagulation for cancer patients with VTE.
Treatment of portal vein trombosis in pancreatic disease
One of questions behind one study was if—and then how—thrombosis after surgery in the portal vein system should be treated (49). Generally, systemic anticoagulation is administered for portal vein thrombus patients without severe obstructive signs of the portal vein system, such as bowel wall edema. For severe occlusion, thrombectomy might be performed immediately as a second surgery. The importance of expeditious treatment with systemic anticoagulation is widely recognized for venous thrombosis in general, and recanalization of acute portal venous system thrombosis may occur in most patients following treatment. However, the treatment was not universally effective in all patients in the present series. The patients in our series received in most cases 5,000-15,000 U LWMHs after detection of portal venous system thrombosis, if anticoagulants were given (49). Increased dosage of LWMHs might become a beneficial future option, but in this limited series of patients we cannot say that patients receiving LMWH were doing better than those that did not—and of course we did not do this in a randomized fashion. One problem was that we did not know when the thrombus started, as the debut symptoms most often were vague, and in most cases the thrombus was found at a computed tomography done in search of a postoperative abscess or in a scheduled follow-up for oncologic reasons. It might be that we detected thrombi that were weeks or months old, where it is least probable that heparin has any positive effect. Therefore, we still question if we ought to treat portal vein thrombosis in the follow-up after pancreatic resections, although we must admit that we still do it if there are indications that it is a newly formed thrombus, as in the first postoperative period. Recent and ongoing clinical trials have focused on VTE prophylaxis with LMWH in high-risk cancer outpatients, particularly those with pancreatic cancer. These studies have yielded encouraging preliminary results but whether thromboprophylaxis provides significant benefit to unselected cancer outpatients remains unclear. A risk stratification incorporating known risk factors and biomarkers may identify those patients at highest risk (42,54,65).
Treatment of pulmonary embolus in pancreatic disease
LMWH is the preferred initial therapy for VTE. Until further data emerge, thrombolysis and vena cava filters should be reserved for patients in whom anticoagulation is insufficient or contraindicated. Optimal initial management of VTE in patients with cancer entails maintaining a high index of suspicion for thrombotic disease, confirming diagnostic suspicions with objective testing and evidence-based use of anticoagulation, and adjunctive therapeutic modalities (thrombolysis, vena cava interruption, venous stenting). Further investigation of initial diagnostic and treatment strategies for VTE focusing on patients with cancer are warranted (66).
Conclusions
Thromboembolism is still an uncommon manifestation of pancreatic disease and results from hypercoagulable and inflammatory states, but the pathophysiology is complex and possibly there are both independent and interrelated factors leading to the thromboembolic events.
If the incidence is clinically significantly increased in pancreatic cancer remains to be proven; it may be accepted that the incidence is higher than in most other gastrointestinal malignancies, but this may be due to the advanced stage in most newly diagnosed pancreatic cancers. It may also be understood that the better the treatment is and the longer the patients due to that lives, the higher is the risk for thromboembolism if the patient is not definitely cured from the malignancy. It is not ultimately shown that the prognosis of pancreatic cancer is worse if the patient has a venous thrombosis (even though there are some indications pointing in that direction) or if the thrombosis is better if prophylaxis is given to patients with newly diagnosed disease.
It is not known if patients with portal vein thrombosis due to pancreatic disease or pancreatic surgery should be treated.
Despite the availability of several therapeutic modalities, such as LMWH, thrombolysis and vena cava filter, thromboembolic events associated with pancreatic cancer and both acute and chronic pancreatitis still remain life-threatening. The value of secondary thromboprophylaxis in an unselected cancer or pancreatitis population is uncertain and risk stratification according to risk factors and biomarkers may identify those patients at highest risk where such intervention may be appropriate. Thromboembolic complications, although rare, should be considered in every patient with pancreatic cancer or pancreatitis, as early detection and management may reduce morbidity and mortality.
We have reached further than Trousseau—but not far.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Warren R. Behavior of venous thrombi: historical observations. Arch Surg 1980;115:1151-4. [PubMed]
- Bariéty M. Tribute to Armand Trousseau (14 October 1801-23 June 1867). Bull Acad Natl Med 1967;151:627-35. [PubMed]
- Pinzon R, Drewinko B, Trujillo JM, et al. Pancreatic carcinoma and Trousseau’s syndrome: experience at a large cancer center. J Clin Oncol 1986;4:509-14. [PubMed]
- Sack GH Jr, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 1977;56:1-37. [PubMed]
- Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 2007;110:1723-9. [PubMed]
- Molacek J, Treska V, Baxa J, et al. Phlegmasia cerulea dolens as a complication of a severe form of acute hemorrhagic-necrotizing pancreatitis. Blood Coagul Fibrinolysis 2008;19:98-100. [PubMed]
- Gonzelez HJ, Sahay SJ, Samadi B, et al. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB (Oxford) 2011;13:860-4. [PubMed]
- Zhang Q, Zhang QX, Tan XP, et al. Pulmonary embolism with acute pancreatitis: a case report and literature review. World J Gastroenterol 2012;18:583-6. [PubMed]
- Haertwig S, Holle A, Emmrich J. Course of chronic pancreatitis in the northeast of Germany. Pancreatology 2011;11:146.
- Barbu ST, Cazacu M. Portal vein thrombosis in chronic pancreatitis – prevalence and risk factors. Pancreatology 2011;11:141.
- Adam U, Makowiec F, Riediger H, et al. Pancreatic head resection for chronic pancreatitis in patients with extrahepatic generalized portal hypertension. Surgery 2004;135:411-8. [PubMed]
- Sakorafas GH, Sarr MG, Farley DR, et al. The significance of sinistral portal hypertension complicating chronic pancreatitis. Am J Surg 2000;179:129-33. [PubMed]
- Evans GR, Yellin AE, Weaver FA, et al. Sinistral (left-sided) portal hypertension. Am Surg 1990;56:758-63. [PubMed]
- Agarwal AK, Raj Kumar K, Agarwal S, et al. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg 2008;196:149-54. [PubMed]
- Butler JR, Eckert GJ, Zyromski NJ, et al. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford) 2011;13:839-45. [PubMed]
- Heider TR, Azeem S, Galanko JA, et al. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg 2004;239:876-80; discussion 880-2. [PubMed]
- Seeger M, Günther R, Hinrichsen H, et al. Chronic portal vein thrombosis: transcapsular hepatic collateral vessels and communicating ectopic varices. Radiology 2010;257:568-78. [PubMed]
- Stein GY, Schwartz A, Neuman H, et al. Bilateral deep vein thrombosis as presenting symptom of malignancy. Harefuah 2007;146:800-2,812. [PubMed]
- Kessler CM. The link between cancer and venous thromboembolism: a review. Am J Clin Oncol 2009;32:S3-7. [PubMed]
- Sørensen HT, Sværke C, Farkas DK, et al. Superficial and deep venous thrombosis, pulmonary embolism and subsequent risk of cancer. Eur J Cancer 2012;48:586-93. [PubMed]
- Shinagare AB, Guo M, Hatabu H, et al. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer 2011;117:3860-6. [PubMed]
- White RH, Chew H, Wun T. Targeting patients for anticoagulant prophylaxis trials in patients with cancer: who is at highest risk? Thromb Res 2007;120:S29-40. [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007;110:2339-46. [PubMed]
- Heidrich H, Konau E, Hesse P. Asymptomatic venous thrombosis in cancer patients--a problem often overlooked. Results of a retrospective and prospective study. Vasa 2009;38:160-6. [PubMed]
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer 2010;103:947-53. [PubMed]
- Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest 2009;27:63-74. [PubMed]
- Dumitrascu DL, Suciu O, Grad C, et al. Thrombotic complications of pancreatic cancer: classical knowledge revisited. Dig Dis 2010;28:350-4. [PubMed]
- Davidson T, Goitein O, Avigdor A, et al. 18F- FDG-PET/CT for the diagnosis of tumor thrombosis. Isr Med Assoc J 2009;11:69-73. [PubMed]
- Boccaccio C, Comoglio PM. Genetic link between cancer and thrombosis. J Clin Oncol 2009;27:4827-33. [PubMed]
- Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 2005;105:1734-41. [PubMed]
- Khorana AA, Ahrendt SA, Ryan CK, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res 2007;13:2870-5. [PubMed]
- van den Berg YW, Osanto S, Reitsma PH, et al. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 2012;119:924-32. [PubMed]
- Poruk KE, Firpo MA, Huerter LM, et al. Serum platelet factor 4 is an independent predictor of survival and venous thromboembolism in patients with pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev 2010;19:2605-10. [PubMed]
- Westrick RJ, Eitzman DT. Plasminogen activator inhibitor-1 in vascular thrombosis. Curr Drug Targets 2007;8:966-1002. [PubMed]
- Andrén-Sandberg A, Lecander I, Martinsson G, et al. Peaks in plasma plasminogen activator inhibitor-1 concentration may explain thrombotic events in cases of pancreatic carcinoma. Cancer 1992;69:2884-7. [PubMed]
- Kaur S, Kumar S, Momi N, et al. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol 2013;10:607-20. [PubMed]
- Shao B, Wahrenbrock MG, Yao L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 2011;118:4015-23. [PubMed]
- Wahrenbrock M, Borsig L, Le D, et al. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 2003;112:853-62. [PubMed]
- Li YH, Kuo CH, Shi GY, et al. The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci 2012;19:34. [PubMed]
- Caine GJ, Stonelake PS, Lip GY, et al. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 2002;4:465-73. [PubMed]
- Boccaccio C, Sabatino G, Medico E, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature 2005;434:396-400. [PubMed]
- Kar SP, Bhosale P, Overman MJ, et al. Role of anticoagulation (AC) in patients with advanced pancreatic cancer (APC) with splanchnic venous thrombosis (SVT). 2011 ASCO Annual Meeting. Chicago, IL, USA, 2011.
- Epstein AS, Soff GA, Capanu M, et al. Analysis of incidence and clinical outcomes in patients with thromboembolic events and invasive exocrine pancreatic cancer. Cancer 2012;118:3053-61. [PubMed]
- Menapace LA, Peterson DR, Berry A, et al. Symptomatic and incidental thromboembolism are both associated with mortality in pancreatic cancer. Thromb Haemost 2011;106:371-8. [PubMed]
- Mandala M, Labianca R, Agnelli G, et al. Multicenter, prospective, case-control, observational study on the influence of venous thromboembolism on patient outcome. 2011 ASCO Annual Meeting. Chicago, IL, USA, 2011.
- Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334-49. [PubMed]
- Shaib W, Deng Y, Zilterman D, et al. Assessing risk and mortality of venous thromboembolism in pancreatic cancer patients. Anticancer Res 2010;30:4261-4. [PubMed]
- Mitry E, Taleb-Fayad R, Deschamps A, et al. Risk of venous thrombosis in patients with pancreatic adenocarcinoma. Gastroenterol Clin Biol 2007;31:1139-42. [PubMed]
- Ansari D, Ansorge C, Andrén-Sandberg A, et al. Portal venous system thrombosis after pancreatic resection. World J Surg 2013;37:179-84. [PubMed]
- Kim AW, McCarthy WJ 3rd, Maxhimer JB, et al. Vascular complications associated with pancreaticoduodenectomy adversely affect clinical outcome. Surgery 2002;132:738-44; discussion 744-7. [PubMed]
- Smoot RL, Christein JD, Farnell MB. Durability of portal venous reconstruction following resection during pancreaticoduodenectomy. J Gastrointest Surg 2006;10:1371-5. [PubMed]
- Stauffer JA, Dougherty MK, Kim GP, et al. Interposition graft with polytetrafluoroethylene for mesenteric and portal vein reconstruction after pancreaticoduodenectomy. Br J Surg 2009;96:247-52. [PubMed]
- Ansari D, Ansorge C, Andrén-Sandberg A, et al. Thromboembolism after pancreatic resection. Pancreatology 2010;10:392.
- Menapace LA, Khorana AA. The role of thromboprophylaxis in cancer patients: emerging data. Curr Opin Hematol 2010;17:450-6. [PubMed]
- Mandalà M, Moro C, Labianca R. Venous thromboembolism and pancreatic cancer: incidence, pathogenesis and clinical implications. Onkologie 2008;31:129-35. [PubMed]
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53. [PubMed]
- Piovella F, Crippa L, Barone M, et al. Normalization rates of compression ultrasonography in patients with a first episode of deep vein thrombosis of the lower limbs: association with recurrence and new thrombosis. Haematologica 2002;87:515-22. [PubMed]
- Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955-60. [PubMed]
- Kearon C. Natural history of venous thromboembolism. Circulation 2003;107:I22-30. [PubMed]
- Budman DR, Barginear MF, Gralla RJ, et al. Documenting the complete resolution rates of venous thromboemboli (VTE) with the factor Xa inhibitor fondaparinux sodium (FS) in patients with cancer. 2011 ASCO Annual Meeting. Chicago, IL, USA, 2011.
- von Delius S, Ayvaz M, Wagenpfeil S, et al. Effect of low-molecular-weight heparin on survival in patients with advanced pancreatic adenocarcinoma. Thromb Haemost 2007;98:434-9. [PubMed]
- Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012;48:1283-92. [PubMed]
- Riess H, Pelzer U, Opitz B, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy: Final results of the CONKO-004 trial. 2010 ASCO Annual Meeting. Chicago, IL, USA, 2010.
- Barginear MF, Gralla RJ, Akerman M, et al. Is there an advantage to adding inferior vena cava filter (IVCF) placement to anticoagulation with fondaparinux in patients with cancer and venous thromboemboli (VTE): Results of the Cancer and Thrombosis (CAT) prospective randomized clinical trial (RCT). 2011 ASCO Annual Meeting. Chicago, IL, USA, 2011.
- Leonardi MJ, Hollander LL, Pitt HA, et al. Acute Portomesenteric Venous Thrombosis Following Abdominal Surgery: Observe, Anticoagulate or Operate? 52nd Annual Meeting. Chicago, Illinois, USA, 2011.
- Streiff MB. Diagnosis and initial treatment of venous thromboembolism in patients with cancer. J Clin Oncol 2009;27:4889-94. [PubMed]


