Deletion of tumor progression locus 2 attenuates alcohol-induced hepatic inflammation
Introduction
Excess consumption of alcohol is widespread throughout the world and chronic intoxication of alcohol is known to cause serious organ damage. The Center for Disease Control reports that as of 2011, over 50% of the United States population consumes alcohol (1). Of this population, 6.2%—almost 16 million individuals—are categorized with heavy alcohol use (defined as five or more drinks on the same occasion on each of 5 or more days in the past 30 days) and over 23% binge drink (defined as five or more drinks on the same occasion on at least 1 day in the past 30 days) (1). Specifically, alcohol consumption is one of the most prominent factors contributing to liver disease—a major cause of morbidity and mortality worldwide (2,3). The initial development of alcoholic liver disease (ALD) is characterized by hepatosteatosis (fatty liver) followed by the development of alcoholic steatohepatitis. The disease state can then further progress to the irreversible stages of fibrosis and cirrhosis, and increase the risk for hepatocellular carcinoma if alcohol consumption is continued (4-6).
The early stages of ALD are characterized by lipid accumulation in the hepatocytes and activation of several inflammatory signaling pathways (6-8). Specifically, consumption of alcohol stimulates lipopolysaccharide (LPS) to enter the circulation from the gut and activate Kupffer cells via toll-like receptor 4 (TLR4) initiating production of inflammatory mediators tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) (9-12). Mitogen-activated protein (MAP) kinases, including extracellular receptor activated kinases 1/2 (ERK1/2) and c-jun-N-terminal kinase (JNK), play an essential signaling role in this inflammatory response (6,10,12-14).
Tumor progression locus 2 (TPL2), also known as Cancer Osaka Thyroid (COT) and MAP3K8, is a serine-threonine kinase that functions as a critical regulator of inflammatory pathways by up-regulating production of inflammatory cytokines. TPL2 functions downstream of IkappaB kinase-β (IKK-β) to activate MAP kinases and up-regulate production of inflammatory cytokines, including IL-6, TNFα, and IL-1β (15). Specifically, TPL2 is known to directly phosphorylates a MAP2K which further activate the ERK1/2, JNK, and to a smaller extent p38 pathways through the mechanism of direct phosphorylation (16,17). TPL2 has been shown to be required for optimal TNFα production by LPS-stimulated macrophages through activation of ERK1/2 in response to TLR4 stimulation. Further, TPL2 knockout (KO) mice were shown to be resistant to LPS-induced pathology, due to low production of TNFα (18). TPL2 also positively regulates mRNA and protein induction of IL-1β following stimulation of macrophages with LPS through TLR-induced ERK activation (19,20). Recently, we have demonstrated that TPL2 KO mice had significantly lower incidence of liver tumors and developed hepatocellular adenoma only, which is contrast to wild-type (WT) mice where they all developed hepatocellular carcinoma (21). In addition, TPL2 KO mice had significantly down-regulated phosphorylation of JNK and ERK, and levels of mRNA expression of pro-inflammatory cytokines, which correlated with the reduced incidence and number of hepatic inflammatory foci (21). These data raise an important question of whether TPL2 plays a role in the mechanism of alcohol-induced inflammation. To our knowledge, this question has not yet been investigated.
This study utilized WT and TPL2KO mice to investigate the role of TPL2 in an alcohol-fed mouse model. Potential alterations of histological and biochemical changes known to be involved in the induction of hepatic inflammation by alcohol consumption were examined in our model.
Methods
Animals and diet
Male TPL2+/+ WT and TPL2−/− KO mice were provided by Dr. Philip Tschilis (Tufts University, Boston, MA) and backcrossed into C57BL/6J mice for >9 generations, as previously described (18,22). Experimental mice were fed a Lieber-DeCarli liquid ethanol diet [EtOH diet, 27% of total calories as EtOH yielding an EtOH concentration of 5% (vol/vol)] or control diet (ctrl diet, where EtOH is replaced by isocaloric amounts of maltodextrin) (Dyets, Inc., Bethlehem, PA, Germany) daily. The Lieber-DeCarli liquid diet conserved the advantage of having all the ingredients resolved in water and food intake is easily monitored, and has been widely used in alcohol research. The Lieber-DeCarli Liquid Diet meets the National Research Council Committee on Animal Nutrition (NRC) recommendations for murine diets. Following breeding, mice 10–20 weeks of age were randomized into four groups. Groups include: (I) WT + ctrl diet; (II) TPL2KO + ctrl diet; (III) WT + EtOH diet; and (IV) TPL2KO + EtOH diet. Following an adaptation to the ctrl diet for one week, the EtOH diet-fed group was allowed free access to diet containing 1% (vol/vol) EtOH followed by gradually increasing concentrations of EtOH over a two week adaptation period. Mice were then group-pair fed the full 5% (vol/vol) EtOH or ctrl diet for a 4-week treatment period. Mice were group pair-fed with EtOH diet as lead group for the duration of the animal protocol. Animal growth was monitored via weekly weighing and dietary intake was assessed daily. As the Lieber-DeCarli liquid diets provide sufficient amounts of water, no extra fluids were given for the duration of the studies. Upon completion of the study timeline, animals were terminally exsanguinated under deep isoflurane anesthesia. All animal protocols and procedures were done under the approval of the Institutional Animal Care and Use Committee at the USDA Human Nutrition Research Center on Aging at Tufts University.
Quantification of hepatic inflammatory foci
Formalin-fixed and paraffin-embedded liver tissue was routinely processed for hematoxylin and eosin (H&E, Sigma Aldrich) staining. The severity of liver inflammation was estimated by the number of inflammatory foci present in each sample, as previously described (23). Briefly, foci were counted on 20 separate fields of view at 100× magnification by two separate blinded investigators for each sample. The mean number of foci was calculated for each group and then compared. A ZEISS microscope with a PixeLINK USB 2.0 (PL-B623CU) digital Camera and PixeLINK µScope Microscopy Software was used for image capturing.
Quantification of steatosis
Formalin-fixed and paraffin-embedded liver tissue was routinely processed for H&E staining. The degree of steatosis was analyzed by capturing 20 images at 100× magnification, as previously described (24). Briefly, images were blindly evaluated by two separate investigators regarding the grade of steatosis (both macro- and micro-vesicular) using a 0–4 grading system based on the percentage of the liver section that is occupied by fat vacuoles (grade 0 ≤5% steatosis; grade 1 =5–25%; grade 2 =26–50%; grade 3 =51–75%; grade 4 ≥75%). A ZEISS microscope with a PixeLINK USB 2.0 (PL-B623CU) digital Camera and PixeLINK µScope Microscopy Software was used for image capturing. Hepatic triglyceride concentrations were measures using the commercial Wako L-type TG M kit (Wako Diagnostics, Richmond, VA, USA) and expressed per µg protein.
RNA extraction and real time-PCR
RNA was isolated using TriPure Isolation Reagent (Roche) as per manufacturer’s instructions, with minor changes. cDNA was then synthesized from RNA samples using M-MLV reverse transcriptase (Invitrogen). Real-time PCR reactions were carried out using SYBR green (Fast Start Universal SYBR Green Master, Roche). Specific primers used are as follows: TPL2 (forward, AAACCAGAGCCGATGTTCCT; reverse, TCGACCACAAGAATGTGGAA), TNFα (forward, CAAACCACCAAGTGGAGGAG; reverse, CGGACTCCGCAAAGTCTAAG), IL-6 (forward, GGATACCACTCCCAACAGACCT; reverse GCCATTGCACAACTCTTTTCTC), IL-1β (forward, TCTTTGAAGTTGACGGACCC; reverse, TGAGTGATACTGCCTGCCTG), MCP-1 (forward, TCAGCCAGATGCAGTTAACGC; reverse, TCTGGACCCATTCCTTCTTGG), F4/80 (forward, CTTTGGCTATGGGCTTCCAGTC; reverse, GCAAGGAGGACAGAGTTTATCGTG), TLR4 (forward, TGTCATCAGGGACTTTGCTG; reverse, TGTTCTTCTCCTGCCTGACA), and GAPDH (forward, CTGGAGAAACCTGCCAAGTATG; reverse, TGAAGTCGCAGGAGACAACCT. Relative changes in gene expression were determined using the −2ΔΔCt method and normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Protein isolation and western blotting
Whole cell liver lysate was extracted and Western blots were performed utilizing 25–50 µg protein as previously described (25). Specific antibodies used include phosphorylated-JNK (Cell Signaling #9251), total-JNK (Cell Signaling #9258), phosphorylated-ERK1/2 (Cell Signaling #9101), and total-ERK1/2 (Cell Signaling #9102). Proteins were detected using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific). Protein bands were quantified using a densitometer (Bio-Rad GS-710, Bio-Rad Laboratories) and phosphorylated protein levels are expressed respective to total protein levels. Protein levels of the housekeeping gene GAPDH (Millipore MAB374) were utilized to ensure equal protein load.
Statistical analyses
Data are represented as means ± SEM. Data were log transformed if needed to obtain normal distribution and statistical differences were determined by t-test and one-way ANOVA with Tukey’s HSD to adjust for multiple comparisons. Kruskal-Wallis overall test followed by Wilcoxon rank-sum test was used for ordinal variables in the steatosis data. All statistical analyses were performed using Statistical Analysis System (SAS®) software; version 9.2 (SAS Institute, Inc., USA) and significance was set at P<0.05.
Results
Effect of TPL2 deletion on final weights of mice fed ctrl and EtOH diets
Male WT and TPL2KO mice were group pair-fed a 27% EtOH or ctrl diet for 4 weeks. As expected, both WT and TPL2KO mice fed EtOH diet had significantly decreased body weights as compared with WT and TPL2KO mice fed ctrl diet (Table 1). WT and TPL2KO mice fed EtOH diet did not have significant changes in liver weight; however, analysis did reveal a significant decrease in ratio of liver weight to body weight, subcutaneous fat, and gonadal fat and a significant increase in fat (both subcutaneous and gonadal) as a percent of body weight as compared with WT and TPL2KO mice fed ctrl diet. Surprisingly, TPL2KO mice fed ctrl diet had significantly decreased body weight as compared to their WT controls. These mice also had significantly decreased liver, subcutaneous fat, and gonadal fat weights as compared with their WT controls. However, no significance was found for liver weight or fat weight as a percent of body weight. No differences were found when comparing WT and TPL2KO mice fed EtOH diet for any of the measured outcomes.
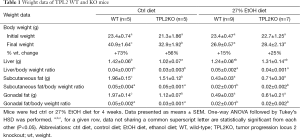
Full table
Mice were fed ctrl or 27% EtOH diet for 4 weeks. Data presented as means ± SEM. One-way ANOVA followed by Tukey’s HSD was performed. a,b,c, for a given row, data not sharing a common superscript letter are statistically significant from each other (P<0.05). Abbreviations: ctrl diet, control diet; EtOH diet, ethanol diet; WT, wild-type; TPL2KO, tumor progression locus 2 knockout; wt, weight.
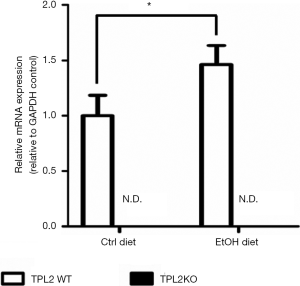
EtOH diet increases hepatic TPL2 expression
WT mice fed EtOH diet for 4 weeks had a significant increase (~1.5 fold, P=0.006) in hepatic Tpl2 mRNA expression as compared with WT mice fed ctrl diet (Figure 1). As expected, hepatic Tpl2 expression was not detected in TPL2KO mice fed either ctrl or EtOH diet.
TPL2 deletion reduces hepatic steatosis in ctrl diet-fed mice
The effects of TPL2 deletion on hepatic steatosis were examined by hepatic triglyceride concentrations and histological analysis of H&E stained slides. Analysis of hepatic triglyceride concentrations revealed 73.1±0.01 µg/µg protein and 72.1±0.01 µg/µg protein in ctrl and EtOH diet fed WT mice, respectively. TPL2KO mice had concentrations of 62.5±0.02 µg/µg protein and 67.7±0.01 µg/µg protein in ctrl and EtOH diet fed mice, respectively. No significant differences were seen among any of the experimental animal groups. Histological analysis of H&E stained slides reveals a significant induction of hepatic steatosis in WT mice fed EtOH diet (Table 2). The median value of the steatosis score increased from 2 to 4 with the consumption of the EtOH diet in WT mice. TPL2KO mice fed ctrl diet had significantly reduced liver steatosis as compared with WT mice with 60% of mice with a score of 0 compare with none in the WT group. TPL2KO mice fed EtOH diet had a nonsignificant reduction in the median value of liver steatosis as compared with WT mice, decreasing from a score of 4 to a score of 2.
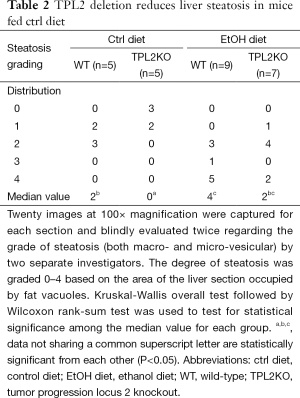
Full table
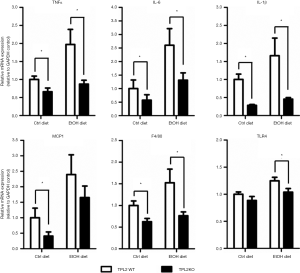
TPL2 deletion reduces hepatic inflammatory foci and cytokine expression
Consumption of the EtOH diet significantly increased inflammatory foci in WT and TPL2KO mice by approximately 2 and 2.4 fold, respectively, as compared to mice fed ctrl diet (Figure 2). Ablation of TPL2 significantly reduced hepatic inflammatory foci in mice fed EtOH diets (~2 fold reduction) and in those fed the ctrl diets (~2.5 reduction) diets as compared to their relative WT controls (P<0.05). Consistent with these histological changes, hepatic expression of inflammatory cytokines TNFα, IL-6, and IL-1β as well as the macrophage marker F4/80 were significantly decreased in TPL2KO mice fed both ctrl and EtOH diets as compared to their respective WT controls (P<0.05). Hepatic mRNA expression is detailed in Figure 3.

Effect of TPL2 deletion on hepatic MAP kinase activity
Potential alterations in MAP kinase signaling were investigated through Western blot analysis. MAP kinase activation was examined through phosphorylated levels relative to total protein levels of ERK1/2 and JNK for each experimental animal group. No significant differences were found between four groups (n=5–9) analyzed for phosphorylated-ERK1/2 (WT ctrl diet, 1.0±0.30; TPL2KO ctrl diet, 0.92±0.19; WT EtOH diet, 1.50±0.42; and TPL2KO EtOH diet, 1.21±0.24) or phosphorylated-JNK (WT ctrl diet, 1.0±0.36; TPL2KO ctrl diet, 0.68±0.31; WT EtOH diet, 1.68±0.44; and TPL2KO EtOH diet, 0.91±0.38).
Discussion
The broad spectrum of injury to the liver, including the induction of inflammation, that is brought on by chronic and excessive alcohol consumption has been well established. However, the exact molecular mechanisms behind this injury have yet to be fully elucidated. Our study, for the first time, utilized TPL2KO mice in an alcohol-fed mouse model to clearly demonstrate the involvement of TPL2 in alcohol-induced hepatic inflammation. Although the mechanism(s) is unclear, importantly, hepatic Tpl2 mRNA expression significantly increased in WT mice fed EtOH diet, further supporting the proposed role of TPL2 in the pathogenesis of ALD. As expected, consumption of the EtOH diet significantly decreased final body weights and fat composition in both WT and TPL2KO mice in our study. Surprisingly, significant changes including a decrease in final body weight, liver weight, and fat (subcutaneous and gonadal) weight were found in TPL2KO mice fed ctrl diet as compared with WT mice fed ctrl diet. However, no changes were found between WT and TPL2KO mice fed EtOH diet in any of the final weight outcomes. Although we cannot explain the differences between our TPL2KO and WT mice that are seen in our ctrl vs. EtOH diets, it is important to note that the observed alterations in hepatic injury outcomes in our alcohol model seen between WT and TPL2KO mice cannot be explained by changes in weight outcomes.
An increased presence of hepatic inflammatory foci, characterized by clusters of recruited immune cells, is a well-known histological feature of ALD. In this study we have shown that TPL2 plays a crucial role in the development of alcohol-induced hepatic inflammation. Our study revealed that the absence of TPL2 significantly reduces the number of hepatic inflammatory foci in mice consuming both ctrl and EtOH diet as compared to their WT controls. This observation was associated with a decrease in hepatic expression of inflammatory cytokines TNFα, IL-6, and IL-1β and the macrophage marker F4/80. Our result is in agreement with a previous study showing that TPL2 KO mice exhibited reduced hepatic injury after acetaminophen challenge, as evidenced by decreased serum levels of both alanine and aspartate aminotransferases, decreased hepatic necrosis, and increased survival relative to WT mice (26). On the other hand, it has been shown that luteolin, one of the common phytonutrients present in celery, parsley, broccoli and herbal spices, could target TPL2 and inhibited its activity in vitro (27). Interestingly, our recent in vivo study has shown that luteolin treatment can inhibit alcohol-promoted, a carcinogen-initiated hepatic carcinogenesis in mice (28). The dramatic reduction in alcohol-induced hepatic inflammation with loss of TPL2 in the present study provided a strong supporting evidence for TPL2 as a new potential target for treatment of ALD.
TPL2 functions to activate downstream signaling of both ERK1/2 and JNK pathways via phosphorylation. Indeed, our recent study showed that there was a significant decrease in the phosphorylation of JNK1/2 and ERK1/2 in TPL2KO mice as compared with WT mice (21). Therefore, we were interested in the investigation of the impact of TPL2 deletion on the phosphorylation of ERK1/2 and JNK in our alcohol-induced hepatic injury model. Unexpectedly, we were not able to see a statistically significant difference in the phosphorylation levels of either ERK1/2 or JNK in mice fed with alcohol diet, as compared with the control diet. Although this is in agreement with the observation reported by Perfield et al. in liver tissue of TPL2KO mice in an obesity model (22), it is possible that our sample size is relative small. In addition, a previous study found expression of TPL2 was significantly reduced in hepatocytes as compared to Kupffer and hepatic stellate cells (29). As hepatocytes are the major cell type found in the liver, this could explain the lack of significance found in downstream signaling of phosphorylated-ERK1/2 and phosphorylated-JNK and would require analysis of signaling in specific cell types instead of whole liver tissue as was performed.
The present study also investigated the presence of hepatic steatosis in our experimental mice. We demonstrated that the absence of TPL2 significantly reduced hepatic steatosis in mice fed ctrl diet. This is not surprising, as the Lieber-DeCarli ctrl diet contains 35% total calories as fat without enough fiber. Therefore, the presence of low-grade steatosis in our WT ctrl diet mice would be expected. Further, it has been shown that ablation of TPL2 significantly reduces lipid accumulation in mice fed a high fat diet (22), thus supporting our observed results in the ctrl diet. Although the mechanism(s) of this phenomenon needs further investigation, we have recently demonstrated that Tpl2 ablation in mice fed with high fat diet resulted in a significant decreased endoplasmic reticulum stress biomarkers, especially in the PERK-eIF2a pathway which is associated with decrease of hepatic steatosis, and the down-regulated protein expression of genes related to do novo lipogenesis, such as ACC and SCD1 (21). We did observe a reduction in the steatosis score of TPL2KO mice fed EtOH diet, but this reduction did not reach statistical significance. However, we believe that this reduction has biological relevance as the median value of steatosis score was reduced from a 4 down to a 2 in these animals. The lack of statistical significance is most likely due to a combination of low sample size and the large variability in in vivo samples and should be addressed using a larger sample size in future studies. To support our steatosis data, hepatic triglyceride concentrations were also analyzed; however no differences between groups were found. It is important to note that there are large regional distributions of steatosis in the liver and the small amount of protein (approximately 50 µg) used for biochemical analysis may not represent the liver as a whole. Therefore, we feel the histological analysis of steatosis is a better representative of the sample and are confident with the results.
In summary, the present study clearly demonstrates the critical role of TPL2 in the pathogenesis of alcohol-induced hepatic inflammation. Specifically, we observe a non-significant reduction in hepatic steatosis and a significant reduction in both the presence of hepatic inflammatory foci and mRNA expression of inflammatory cytokines (TNFα, IL-6, and IL-1β) and macrophage marker F4/80 in TPL2KO mice fed alcoholic diet as compared with WT mice. These data add to our understanding of the molecular mechanisms behind the development of ALD, and provide a potential new avenue for molecular targets for prevention and treatment of ALD.
Acknowledgements
The authors would also like to thank Junrui Cheng for her assistance on this manuscript.
Funding: This study was supported by the USDA/ARS funding No. 58-1950-0014 and No. 58-1950-7-70, and by the NIH/NIDDK (RO1 DK098906, P30-DK-46200, and T32-DK062032).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: Any opinions, findings, conclusions, and recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the sponsors.
Ethical Statement: All animal protocols and procedures for this study were done under the approval of the Institutional Animal Care and Use Committee at the USDA Human Nutrition Research Center on Aging at Tufts University. Animal protocol approval Number was GR-27.
References
- Summary of National Findings: Results from the 2011 National Survey on Drug Use and Health. Rockville, MD, 2011. Available online: http://ncfy.acf.hhs.gov/library/2011/results-2011-national-survey-drug-use-and-health-summary-national-findings
- O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol 2010;105:14-32. [PubMed]
- Williams R. Global challenges in liver disease. Hepatology 2006;44:521-6. [PubMed]
- McCullough AJ, O’Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol 1998;93:2022-36. [PubMed]
- Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology 2004;127:S87-96. [PubMed]
- Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr 2004;24:55-78. [PubMed]
- Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res 2009;33:191-205. [PubMed]
- You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol 2004;287:G1-6. [PubMed]
- Breitkopf K, Nagy LE, Beier JI, et al. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res 2009;33:1647-55. [PubMed]
- Mandrekar P. Signaling mechanisms in alcoholic liver injury: role of transcription factors, kinases and heat shock proteins. World J Gastroenterol 2007;13:4979-85. [PubMed]
- Szabo G, Mandrekar P, Petrasek J, et al. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin Exp Res 2011;35:782-6. [PubMed]
- Valles SL, Blanco AM, Azorin I, et al. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Res 2003;27:1979-86. [PubMed]
- Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol 2009;50:1258-66. [PubMed]
- Min L, He B, Hui L. Mitogen-activated protein kinases in hepatocellular carcinoma development. Semin Cancer Biol 2011;21:10-20. [PubMed]
- Soria-Castro I, Krzyzanowska A, Pelaéz ML, et al. Cot/tpl2 (MAP3K8) mediates myeloperoxidase activity and hypernociception following peripheral inflammation. J Biol Chem 2010;285:33805-15. [PubMed]
- Chiariello M, Marinissen MJ, Gutkind JS. Multiple mitogen-activated protein kinase signaling pathways connect the cot oncoprotein to the c-jun promoter and to cellular transformation. Mol Cell Biol 2000;20:1747-58. [PubMed]
- Salmeron A, Ahmad TB, Carlile GW, et al. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J 1996;15:817-26. [PubMed]
- Dumitru CD, Ceci JD, Tsatsanis C, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 2000;103:1071-83. [PubMed]
- Kaiser F, Cook D, Papoutsopoulou S, et al. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J Exp Med 2009;206:1863-71. [PubMed]
- Mielke LA, Elkins KL, Wei L, et al. Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1 beta production. J Immunol 2009;183:7984-93. [PubMed]
- Li X, Liu C, Ip BC, et al. Tumor progression locus 2 ablation suppressed hepatocellular carcinoma development by inhibiting hepatic inflammation and steatosis in mice. J Exp Clin Cancer Res 2015;34:138. [PubMed]
- Perfield JW 2nd, Lee Y, Shulman GI, et al. Tumor progression locus 2 (TPL2) regulates obesity-associated inflammation and insulin resistance. Diabetes 2011;60:1168-76. [PubMed]
- Veeramachaneni S, Ausman LM, Choi SW, et al. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J Nutr 2008;138:1329-35. [PubMed]
- Wang Y, Seitz HK, Wang XD. Moderate alcohol consumption aggravates high-fat diet induced steatohepatitis in rats. Alcohol Clin Exp Res 2010;34:567-73. [PubMed]
- Chung J, Chavez PR, Russell RM, et al. Retinoic acid inhibits hepatic Jun N-terminal kinase-dependent signaling pathway in ethanol-fed rats. Oncogene 2002;21:1539-47. [PubMed]
- Sanz-Garcia C, Ferrer-Mayorga G, González-Rodríguez Á, et al. Sterile inflammation in acetaminophen-induced liver injury is mediated by Cot/tpl2. J Biol Chem 2013;288:15342-51. [PubMed]
- Kim JE, Son JE, Jang YJ, et al. Luteolin, a novel natural inhibitor of tumor progression locus 2 serine/threonine kinase, inhibits tumor necrosis factor-alpha-induced cyclooxygenase-2 expression in JB6 mouse epidermis cells. J Pharmacol Exp Ther 2011;338:1013-22. [PubMed]
- Rafacho BP, Stice CP, Liu C, et al. Inhibition of diethylnitrosamine-initiated alcohol-promoted hepatic inflammation and precancerous lesions by flavonoid luteolin is associated with increased sirtuin 1 activity in mice. Hepatobiliary Surg Nutr 2015;4:124-34. [PubMed]
- Perugorria MJ, Murphy LB, Fullard N, et al. Tumor progression locus 2/Cot is required for activation of extracellular regulated kinase in liver injury and toll-like receptor-induced TIMP-1 gene transcription in hepatic stellate cells in mice. Hepatology 2013;57:1238-49. [PubMed]


